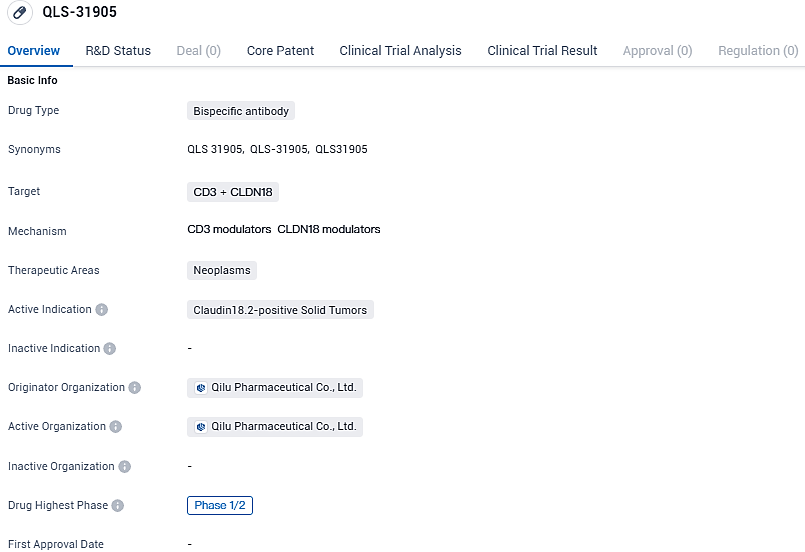
Qilu Pharma Releases Initial Clinical Data on New Dual-Target Antibody QLS31905 During ESMO Immuno-Oncology Meeting
At the ESMO Immuno-Oncology Congress that took place from the 6th to 8th of December in Geneva, Switzerland, Qilu Pharmaceutical revealed recent findings from its Phase I clinical study concerning QLS31905. This study pertains to individuals afflicted with progressive solid malignancies and was showcased via a poster exhibition. Professor Lin Shen of the Peking University Cancer Hospital is at the helm as the principal researcher for this investigation.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Engaged in research and development, Qilu Pharmaceutical has brought forth an innovative bispecific T cell engager called QLS31905, focusing on the target molecule Claudin18.2. This investigation primarily sought to discern the tolerability, safety profile, and initial efficacy against cancer of QLS31905 in patients battling progressive solid malignancies.
Recognized for its distinct presence on the exterior of cells, Claudin18.2 is peculiarly overexpressed in primary stomach cancer and several other malignancies, including those of the pancreas and esophagus. The design rationale behind QLS31905 is its dual binding capability – attaching simultaneously to Claudin18.2 on cancer cells and the CD3 receptor found on T cells. This action is intended to trigger a robust immune response, systematically engaging T cells to destroy and break down cancerous cells.
Key aims for this phase of investigation were identifying the dosage ceiling without triggering adverse toxic reactions and establishing the maximum dose patients could tolerate. Other aspects under review were the medication's safety profile, its dynamics in the body both in terms of pharmacokinetics and pharmacodynamics, and its possible immunogenic effects. In the latter part of the study, the primary concern shifted to the rate at which tumor responses were observable. Secondary concerns entailed gauging the rate at which the disease was held in check, the duration patients remained disease-free, and overall survival statistics.
Up until the midpoint of July 2023, the clinical study had incorporated 52 subjects, with specific numbers indicating 31 individuals with stomach cancer and a dozen with pancreatic cancer. The dose-escalation stage of the trial saw 22 individuals administered with QLS31905 in dosages ranging from 0.5 μg/kg QW to 350 μg/kg QW. The trial is actively continuing with participants now receiving a dose of 500 μg/kg bi-weekly. At this stage, for the dose-expansion, 30 subjects have been given QLS31905, with dosages established at 200 μg/kg QW and 350 μg/kg every second week.
To conclude, QLS31905 has to this point exhibited a promising safety profile and evidence of antitumor efficacy in the treatment of advanced solid tumors. Efforts are underway with the Phase II clinical trials to provide a thorough evaluation of QLS31905's effectiveness and safety for medical use.
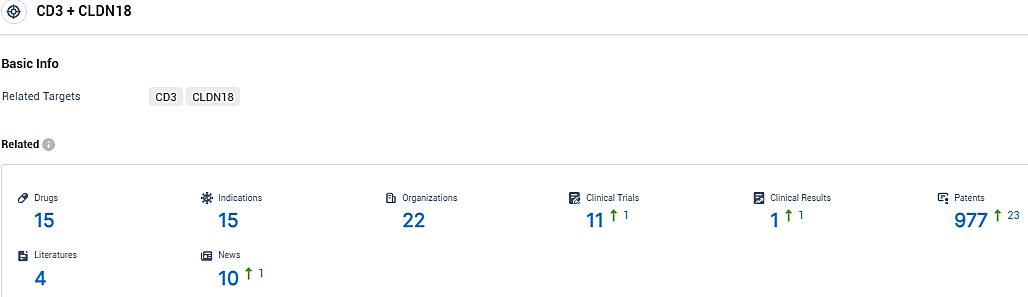
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of December 17, 2023, there are 15 investigational drugs for the CD3 and CLDN18 target, including 15 indications, 22 R&D institutions involved, with related clinical trials reaching 11, and as many as 977 patents.
QLS-31905 targets CD3 and CLDN18 and is primarily focused on treating Claudin18.2-positive solid tumors. The drug is currently in Phase 1/2 of development, indicating that it is still in the early stages of clinical testing. Further research and trials will be needed to determine its safety and efficacy in treating neoplasms.