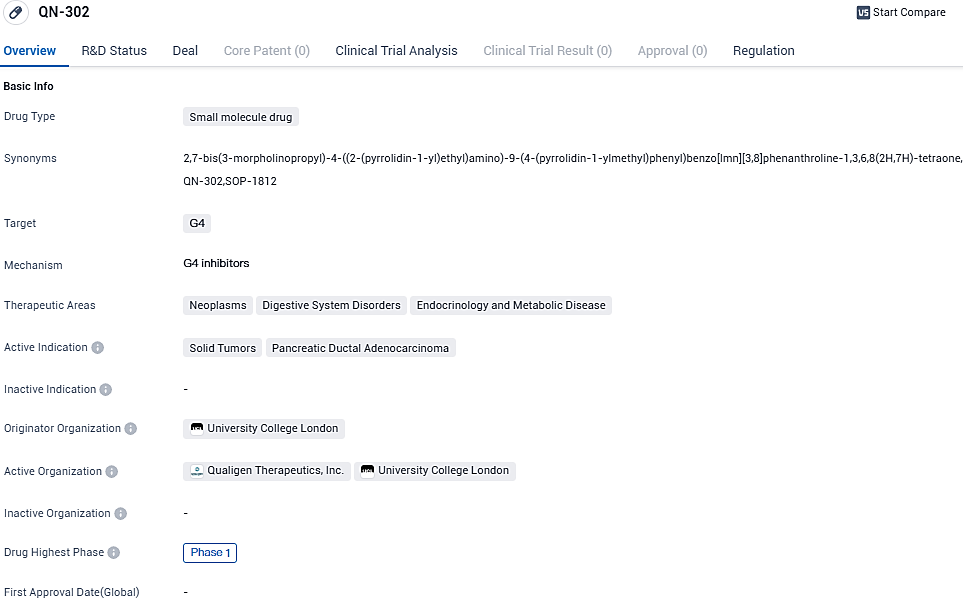
Qualigen Therapeutics announces first dose of Phase 1a QN-302 Clinical Study for advanced solid tumors administered to initial patient
Qualigen Therapeutics, Inc., a firm concentrating on the development of cancer treatments potentially eligible for Orphan Drug Designation, for both adults and children, has disclosed that the inaugural patient of its Phase 1a clinical trial has been administered with QN-302. This given drug could be the first of its kind – a G-Quadruplex (G4)-selective transcription inhibitor under investigation, made exclusively for the advanced or metastasized solid tumors treatment. The initial sequencing of this clinical assessment takes place at START Midwest, Grand Rapids, Michigan.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
QN-302 operates by favoring the stabilization of G4 structures that are common in the promoter region of oncogenes in numerous cancer types, thereby obstructing the transcription of G4-related cancer genes. This could potentially provide a universal approach to cancer treatment, irrespective of the tumor type. For its proposed treatment for pancreatic cancer, Qualigen earned an Orphan Drug Designation in January and was given the green light by the US FDA to commence a Phase 1 clinical trial in July this year.
The presence of G-quadruplexes is noticeable in various challenging tumor types, such as pancreatic cancer," commented Sreenivasa Chandana, M.D., Ph.D., who heads the GI/GU medical oncology research at Cancer and Hematology Centers of Western Michigan in Grand Rapids.
Sreenivasa Chandana further stated, “QN-302 brings forward a possible advancement in the treatment of cancer that could provide a weekly treatment alternative for patients with advanced conditions. Its unique mode of action aims to enhance patient results. We are eager to take part in the premier human clinical trial of QN-302 as a potential new treatment method.”
“The kick-start of the QN-302 Phase 1a clinical trial signals a considerable achievement for Qualigen, propelling us further towards our objective of providing new therapeutic options for devastating diseases such as pancreatic cancer and other complex solid tumors," remarked Tariq Arshad, M.D., M.B.A., Qualigen’s Chief Medical Officer.
Further, Tariq Arshad expressed gratitude towards their CRO, TD2, along with investigation sites, investigators, and every patient and family that are collaboratively exploring the potential of QN-302 as a unique treatment for G4-dominant tumors. He also looks forward to strengthening these partnerships as they enroll more patients into their Phase 1a investigation.
The QN-302 Phase 1a clinical trial will be carried out at a maximum of four top clinics and medical institutions in the US. Apart from the initial site at START Midwest in Grand Rapids, Michigan, they anticipate activating more sites in the Q4 of the current year. Around the first half of 2024, Qualigen expects to release an update on the safety and primary efficiency of the Phase 1a study.
the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
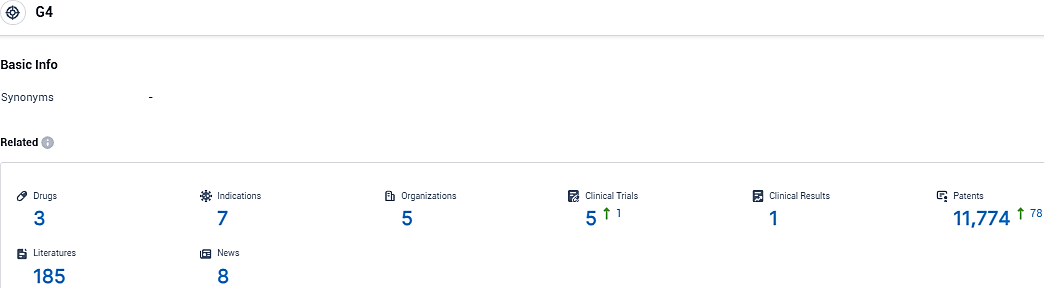
According to the data provided by the Synapse Database, As of November 14, 2023, there are 3 investigational drugs for the G4 target, including 7 indications, 5 R&D institutions involved, with related clinical trials reaching 5, and as many as 11774 patents.
QN-302 is a small molecule G4-selective transcription inhibitor in Phase 1 clinical development for the treatment of G4-expressing solid tumors, such as pancreatic cancer, prostate cancer, sarcomas, and others. Orphan Drug Designation was granted by the FDA in January of this year for QN-302 for the intended indication of pancreatic cancer.