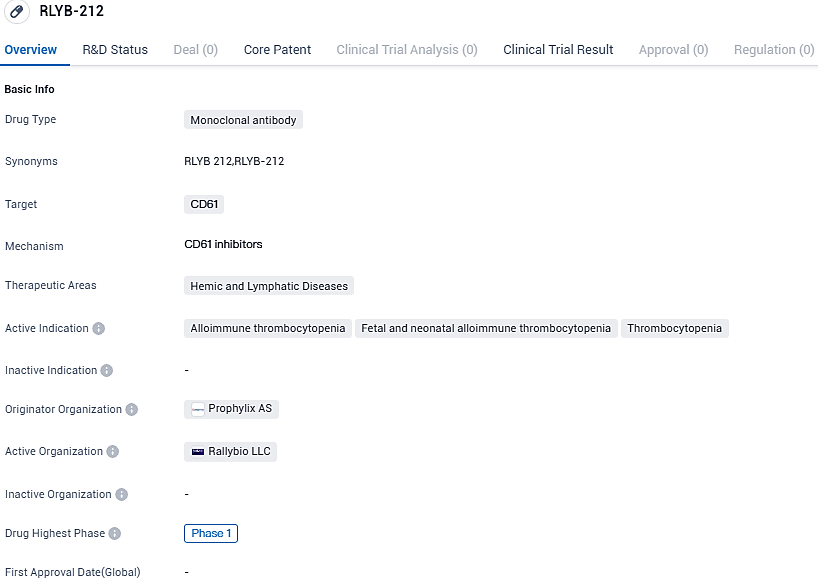
Rallybio Announces Early Results on Safety and Efficacy for RLYB212 Monoclonal Antibody to Prevent Infant Thrombocytopenia from Phase 1 Study
Rallybio Corporation recently disclosed early findings from a concluded group of multiple-dose trials within its Phase 1 evaluation, which is focused on the safety and pharmacokinetic profiles of RLYB212, a monoclonal antibody targeting the prevention of fetal and neonatal alloimmune thrombocytopenia. This targeted antibody specifically acts against HPA-1a.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In early 2023, the initial stage of the RLYB212 trial began, examining the repeated administration's effects on pharmacokinetics (PK) and safety through subcutaneous injections over a three-month period. The study involved a total of eight individuals who do not have the HPA-1a antigen, with six receiving the actual RLYB212 biweekly and the remaining two given a placebo at the same frequency.
The early findings indicated a reliable pharmacokinetic profile following multiple doses, with uniform results noted across and within the participants. These findings, along with predictive clinical pharmacology models, suggest the feasibility of administering RLYB212 once per month, a protocol intended for the upcoming Phase 2 trial. The drug continued to display a favorable safety profile, as per earlier reports, with participants experiencing neither severe adverse reactions nor issues at the injection site.
Rallybio's RLYB212 Program Lead, Dr. Róisín Armstrong, expressed confidence in RLYB212's prophylactic potential to avert HPA-1a alloimmunization and the associated fetal and neonatal alloimmune thrombocytopenia (FNAIT). Armstrong elaborated on the comprehensive completion of the toxicology assessment for RLYB212, which included studies on maternal and fetal toxicity.
Looking ahead, the focus shifts to launching a confirmatory Phase 2 study to evaluate the drug's effectiveness in expectant mothers at an elevated risk of developing FNAIT. This study is slated for the latter half of 2024. Further appreciation was conveyed to the collaborators, including significant contributions from the Fraunhofer Institute for Translational Medicine and Pharmacology and the German Red Cross, in finalizing the Phase 1 research.
In 2024, Rallybio anticipates publishing the details of the RLYB212 pharmacology model, along with the Phase 2 study's dosing strategy and corroborative clinical and preclinical data in a scientific journal subject to peer review.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
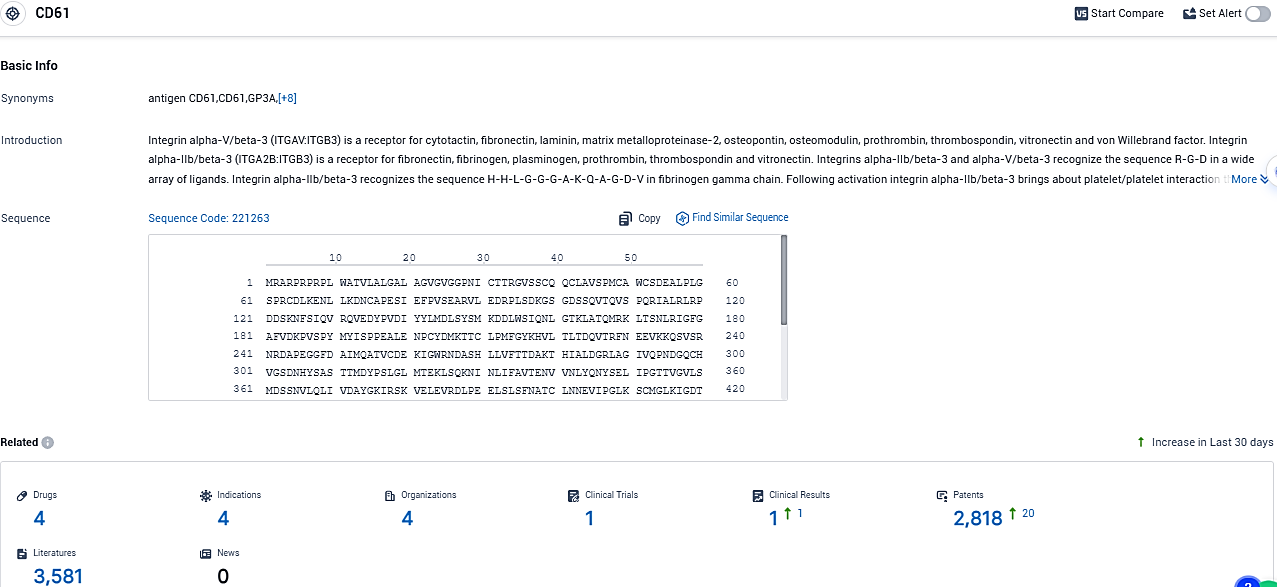
According to the data provided by the Synapse Database, As of December 6, 2023, there are 4 investigational drugs for the CD61 target, including 4 indications, 4 R&D institutions involved, with related clinical trials reaching 1, and as many as 2818 patents.
RLYB-212 is a monoclonal antibody that targets CD61, a protein involved in hemic and lymphatic diseases. The drug is currently in Phase 1 of clinical development and is being evaluated for its safety and potential therapeutic benefits in conditions such as alloimmune thrombocytopenia and thrombocytopenia.