REGENXBIO shares encouraging one-year results from Phase II ALTITUDE trial, using ABBV-RGX-314 for addressing Diabetic Retinopathy through Suprachoroidal Delivery
REGENXBIO Inc. has disclosed supplementary beneficial results from the continuing Phase II ALTITUDE® study. The research involves ABBV-RGX-314, anticipated for managing diabetic retinopathy, excluding center-involved diabetic macular edema, and is administered through in-office suprachoroidal delivery.
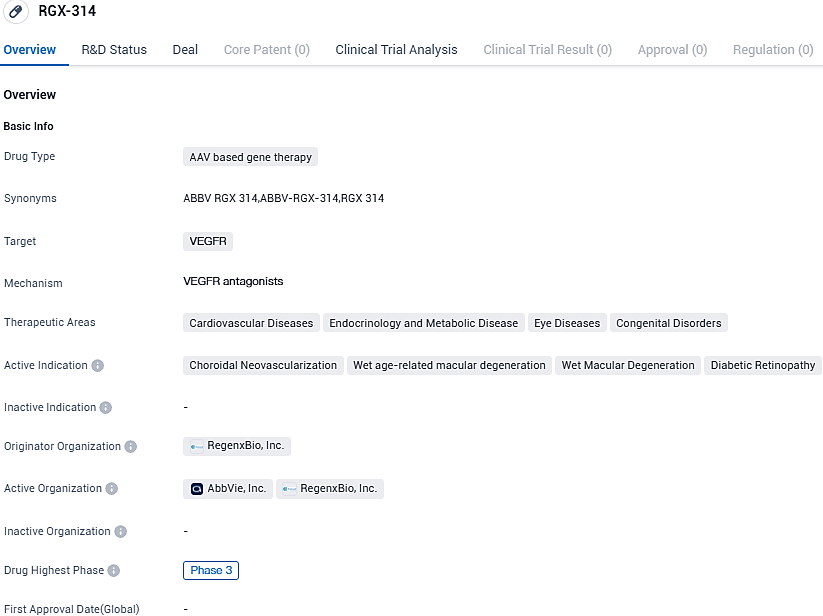
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Retinal Consultants of Arizona's Mark Barakat, M.D. will reveal the data at the American Academy of Ophthalmology assembly in San Francisco, CA. ABBV-RGX-314, the subject of their analysis, is being probed as a potential one-time gene-based therapy for the management of wet age-related macular degeneration, DR, and other persistent conditions affecting the retina.
Steve Pakola, M.D., the primary medical official at REGENXBIO, expressed satisfaction at how ABBV-RGX-314 at dosage level 2 continues to demonstrate significant improvement in patients suffering from non-proliferative DR, along with it being well accepted. He further added that a single, in-practice application of ABBV-RGX-314 can potentially stabilize the DR severity score and decrease the long-standing risk of vision-damaging episodes.
Dr. Barakat referred to DR as a major contributor to vision loss in individuals of working-age worldwide and recognized it as a global health issue. He displayed optimism over the positive results noted in the one-year ALTITUDE trial of ABBV-RGX-314. He highlighted the urgent necessity for long-term treatment options, which can control the initial stages of diabetic retinopathy from developing into proliferative diabetic retinopathy and vision-related complications. He's keenly waiting for additional data from the ALTITUDE trial related to ABBV-RGX-314 one-time treatment.
ABBV-RGX-314, a joint development initiative with AbbVie, is the potential gene therapy being looked at for one-time treatment of wet AMD, diabetic retinopathy, and other lingering retinal disorders.
REGENXBIO is pushing forward research in two separate administration pathways of ABBV-RGX-314 to the eye, through standard subretinal delivery procedure and delivery to the suprachoroidal space. REGENXBIO has procured the exclusive rights for the delivery of gene therapy treatments to the eye's suprachoroidal space via the SCS Microinjector® from Clearside Biomedical, Inc.
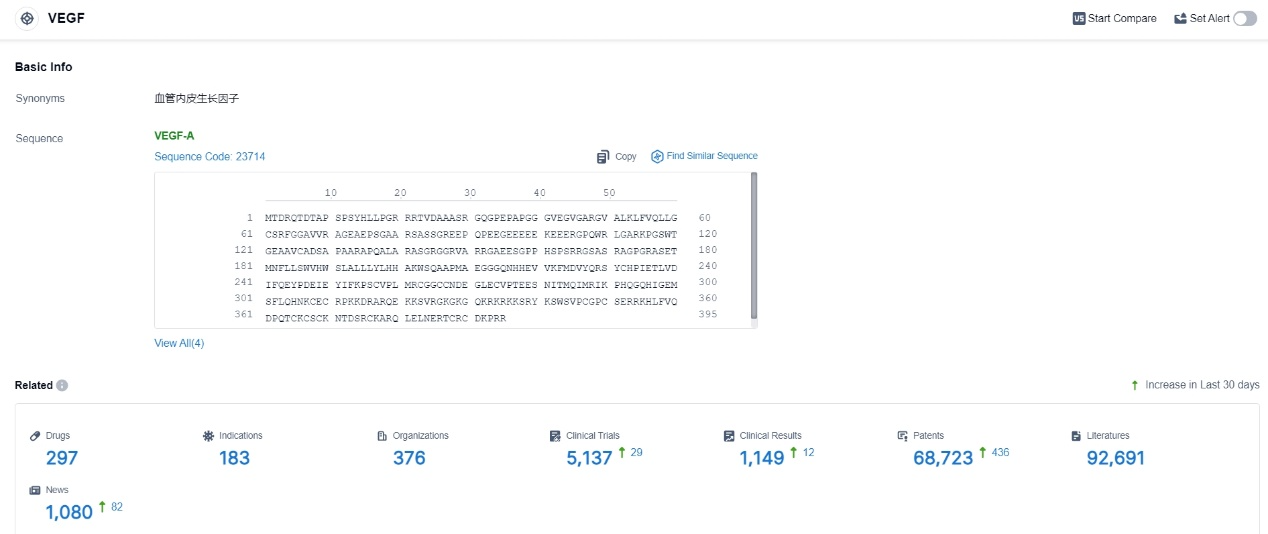
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 11, 2023, there are 297 investigational drugs for the VEGF target, including 183 indications, 376 R&D institutions involved, with related clinical trials reaching 5137, and as many as 68723 patents.
ABBV-RGX-314 consists of the NAV® AAV8 vector, which encodes an antibody fragment designed to inhibit vascular endothelial growth factor (VEGF). ABBV-RGX-314 is believed to inhibit the VEGF pathway by which new, leaky blood vessels grow and contribute to the accumulation of fluid in the retina.