Repare Therapeutics launches two clinical trials by 2024: RP-1664 and RP-3467, both oral inhibitors
Repare Therapeutics Inc., a renowned firm specializing in advanced stage precision oncology, today unveiled polo-like kinase 4 (PLK4) as the focus of its RP-1664 development plan. It also presented in-depth preclinical statistics for both RP-1664 and the Company's Polθ inhibitor, RP-3467.
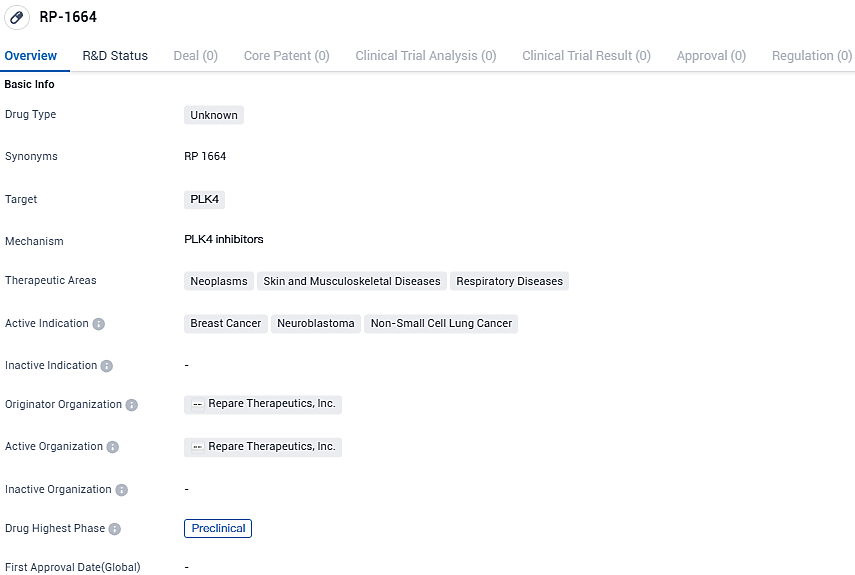
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
RP-1664 is an innovative, best-in-class, oral PLK4 inhibitor that shows synthetic lethality with TRIM37 amplification or an excess of expression in solid tumors. This selectivity is important as tumors rely on PLK4 to survive when TRIM37 is present in high quantities.
Findings from preclinical research illustrate that RP-1664 brings about potent synthetic lethality in models of tumors with high levels of TRIM37, in both lab cultures (in vitro) and living organisms (in vivo). Raised TRIM37 expression is a commonality found in several solid tumors and nearly all high-grade neuroblastomas.
RP-3467, meanwhile, is a leading candidate for inhibiting DNA polymerase theta, also known as Polθ. Polθ is a synthetic lethal target associated with tumors deficient in homologous recombination, like those carrying BRCA1/2 mutations or other genomic alterations.
Existing data propose that RP-3467 acts in concert with treatments that cause double-stranded DNA fractures, including PARP inhibition, radioligand therapy and various chemotherapies and antibody-drug conjugates. Preliminary data also imply that inhibiting Polθ could disrupt processes that are crucial for PARPi resistance development.
CEO and President of Repare, Lloyd M. Segal, announced, "We are thrilled to reveal the initial clinical plan for RP-1664, backed by compelling preclinical data supporting its efficacy in treating high TRIM37 tumors. We are equally delighted to share preliminary data from our RP-3467 program, which highlights its considerable potential across several high-value therapeutic applications. Our plan is to progress both RP-1664 and RP-3467 into Phase 1 clinical trials in 2024."
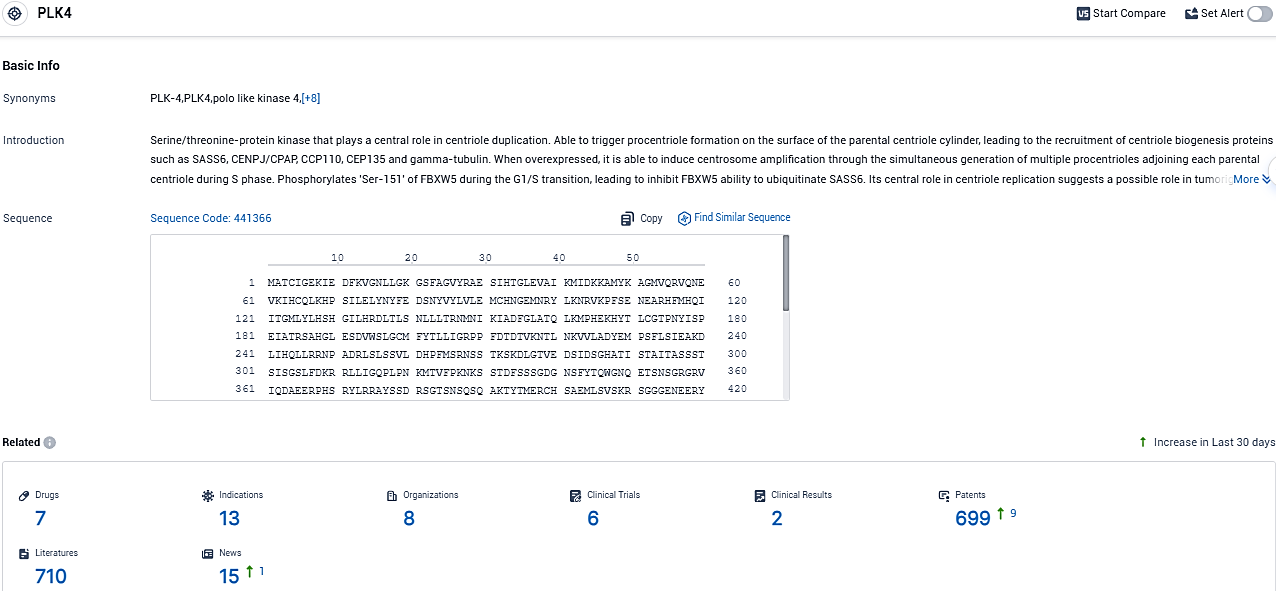
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 22, 2023, there are 7 investigational drugs for the PLK4 target, including 13 indications, 8 R&D institutions involved, with related clinical trials reaching 6 and as many as 699 patents.
RP-1664 is a highly potent, selective and bioavailable PLK4 inhibitor that is synthetic lethal with TRIM37 gain of function. RP-1664 demonstrated robust and dose-dependent monotherapy activity in multiple TRIM37-high preclinical models across a variety of tumor types, including breast cancer, non-small cell lung cancer and neuroblastoma.