Revitalizing Anti-Tumor Immunity and Halting Metastasis by Targeting MS4A4A on Tumor-Associated Macrophages
Immunotherapy has revolutionized cancer treatment, but its effectiveness is often limited by the presence of immunosuppressive myeloid cells, including tumor-associated macrophages (TAMs). TAMs are among the most abundant immune cells observed in colorectal cancer, and their infiltration into the tumor microenvironment has been linked to poor prognosis. TAMs can polarize towards pro-inflammatory M1 or anti-inflammatory M2 phenotypes, and M2-TAMs are known to suppress cytotoxic T cells, leading to T-cell exhaustion.
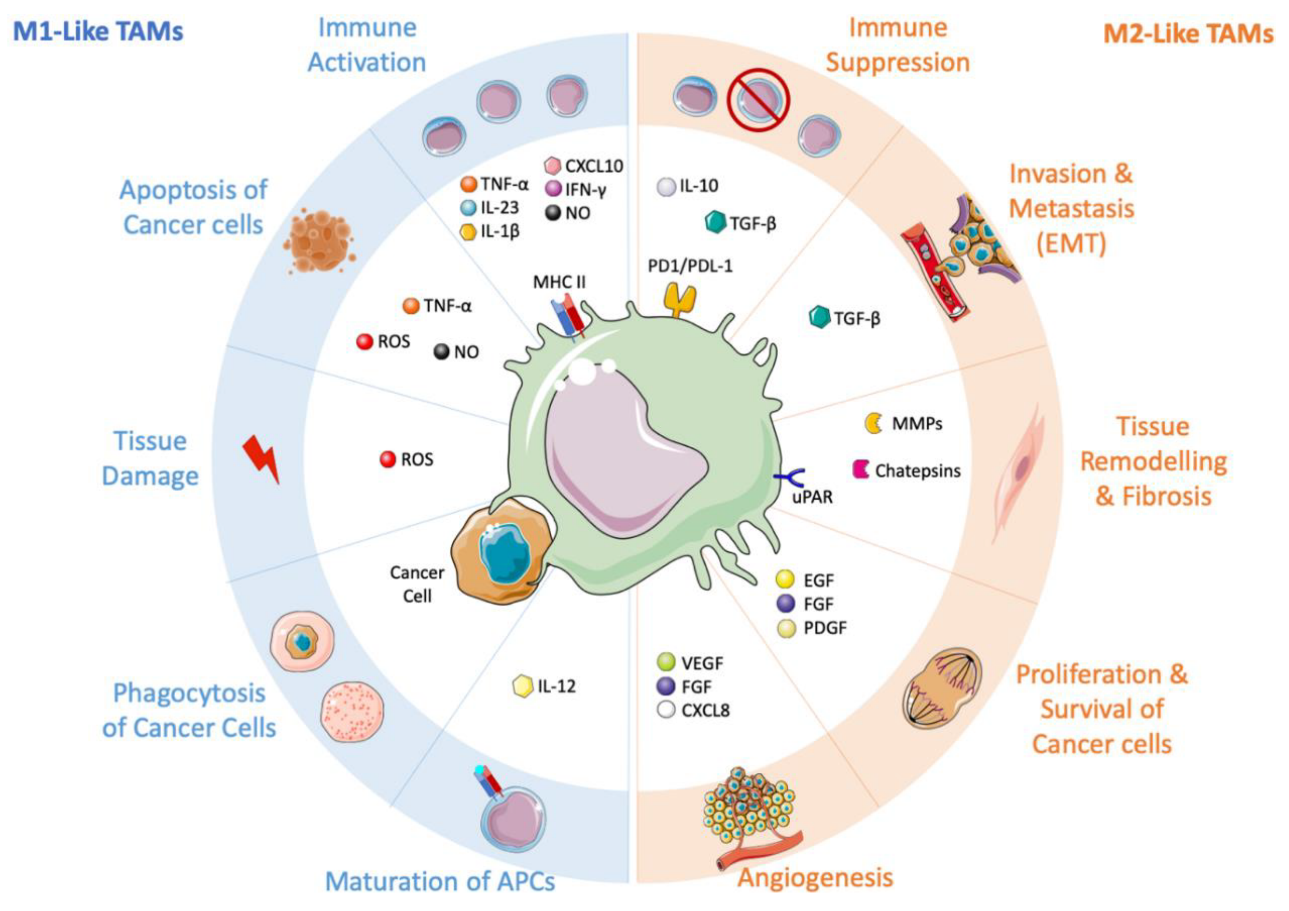
Restoring anti-tumour immunity
A new study published in the journal Gut has revealed a potential new target on TAMs for cancer immunotherapy. The study, led by researchers from Southern Medical University in China, found that targeting a protein called MS4A4A on TAMs could restore CD8+ T-cell-mediated antitumor immunity and enhance the efficacy of immune checkpoint inhibitor (ICI) therapy in colorectal cancer.
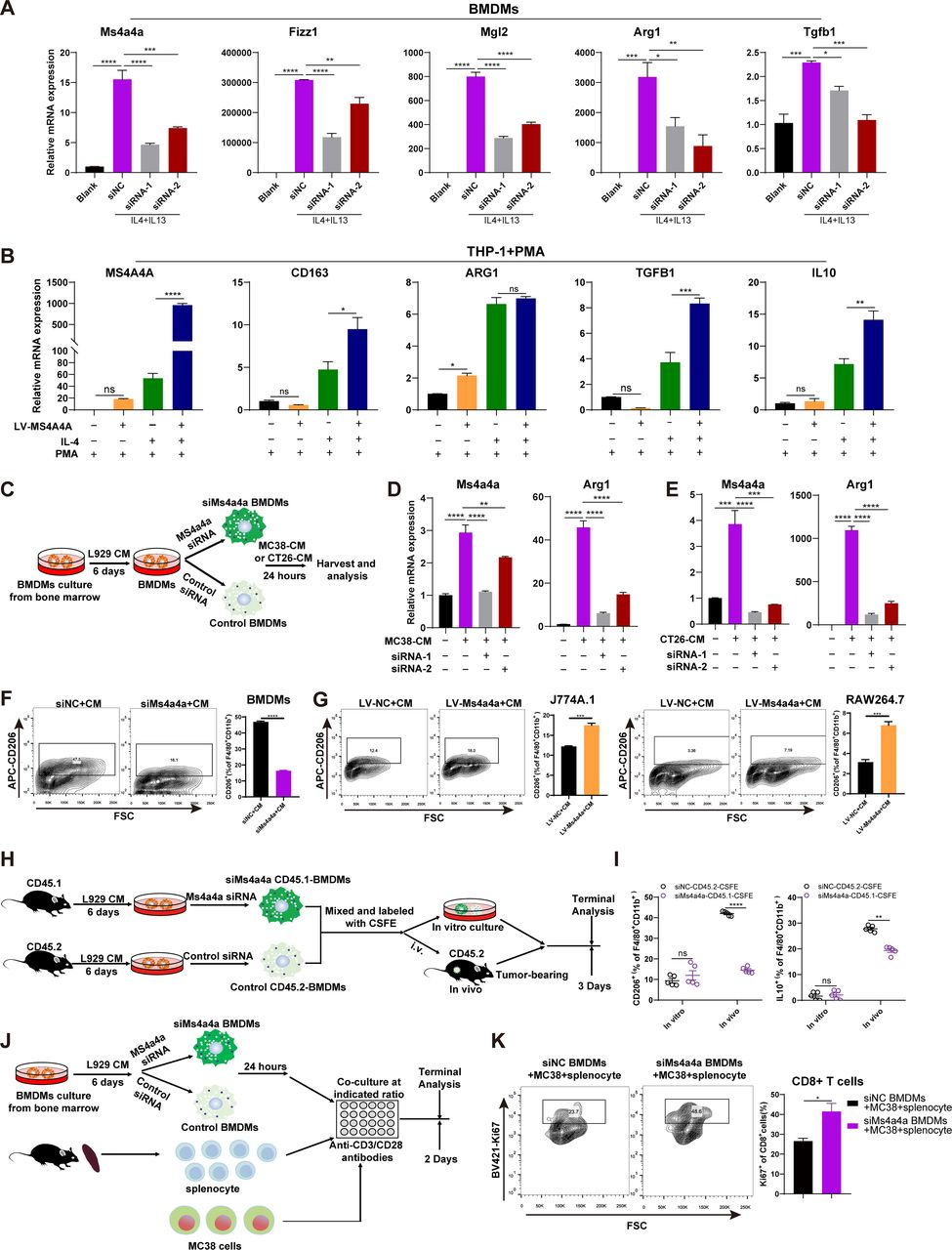
The researchers used public databases and bioinformatic analysis to identify MS4A4A, a transmembrane protein selectively highly expressed in TAMs. They found that MS4A4A promotes M2 polarization of macrophages by activating the PI3K/AKT pathway and JAK/STAT6 pathway, leading to CD8+ T-cell dysfunction.

To investigate the potential of targeting MS4A4A in cancer immunotherapy, the researchers used murine subcutaneous tumour or orthotopic transplanted models and assessed the inhibitory effect of MS4A4A blockade alone or combined with ICI treatment on tumour growth. They found that in vivo inhibition of MS4A4A and anti-MS4A4A monoclonal antibody treatment both curb tumour growth and improve the effect of ICI therapy. MS4A4A blockade treatment reshaped the tumour immune microenvironment, resulting in reducing the infiltration of M2-TAMs and exhausted T cells and increasing the infiltration of effector CD8+ T cells. Anti-MS4A4A plus anti-programmed cell death protein 1 (PD-1) therapy remained effective in large, treatment-resistant tumours and could induce complete regression when further combined with radiotherapy.
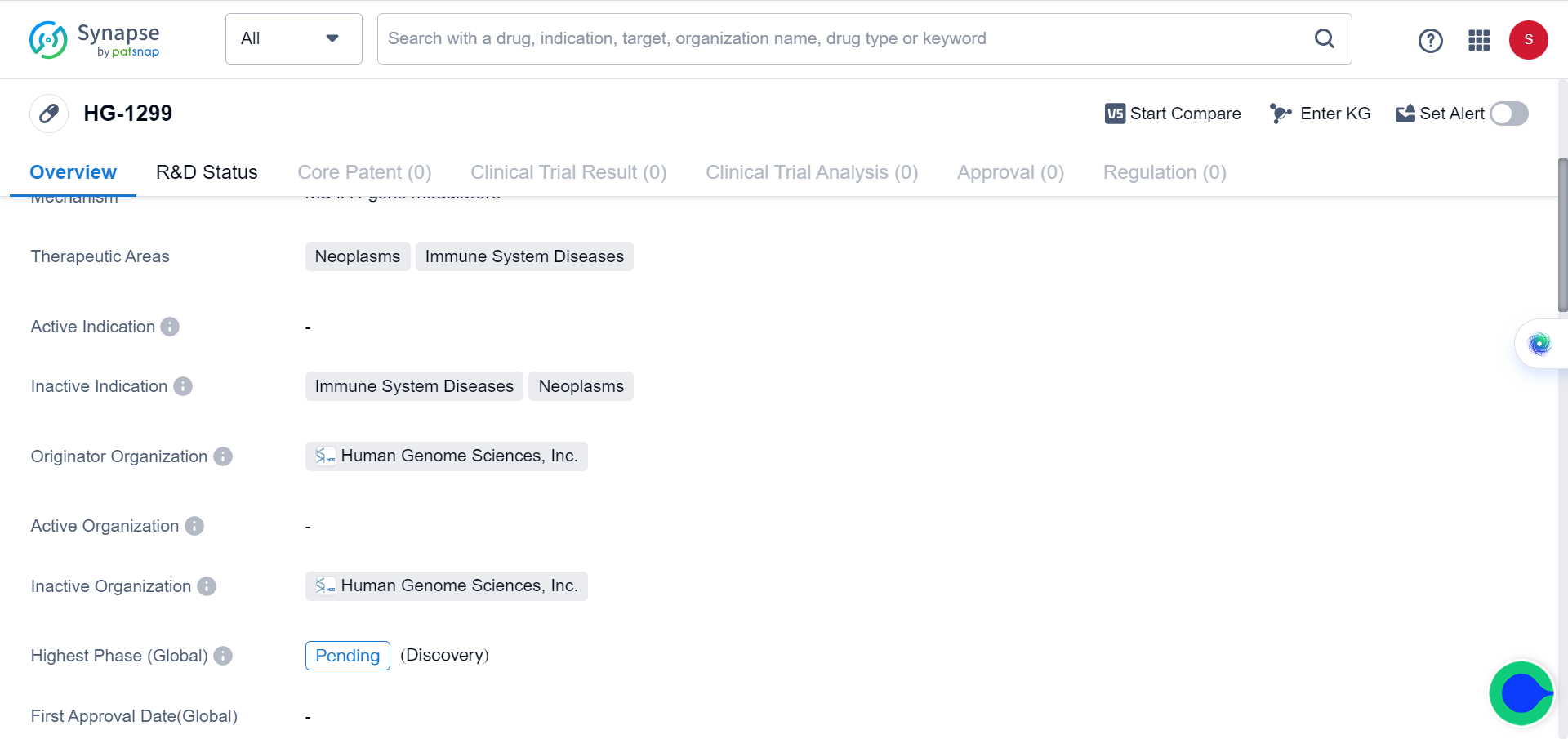
The researchers concluded that targeting MS4A4A could enhance the efficacy of ICI therapy and represent a new anticancer immunotherapy. The Synapse database demonstrates that there’s only one drug (HG-1299) targeting MS4A4A currently available, with the clinical trial status listed as pending, since the role of MS4A4A in immune system’s response to tumor has only recently been elucidated.
MS4A4A enhances NK cell–mediated resistance to metastasis
A previous study published in the journal Nature Immunology has shed light on the role of MS4A4A in the immune system's response to cancer metastasis. The findings suggest that MS4A4A plays a critical role in activating natural killer (NK) cells, which are essential in fighting the spread of cancer cells.
The study was conducted by a team of researchers from the Humanitas Clinical and Research Center in Italy, along with collaborators from several other institutions around the world. Their research focused on the interplay between MS4A4A and Dectin-1, a receptor that is involved in the recognition of fungal pathogens and other microbial invaders.
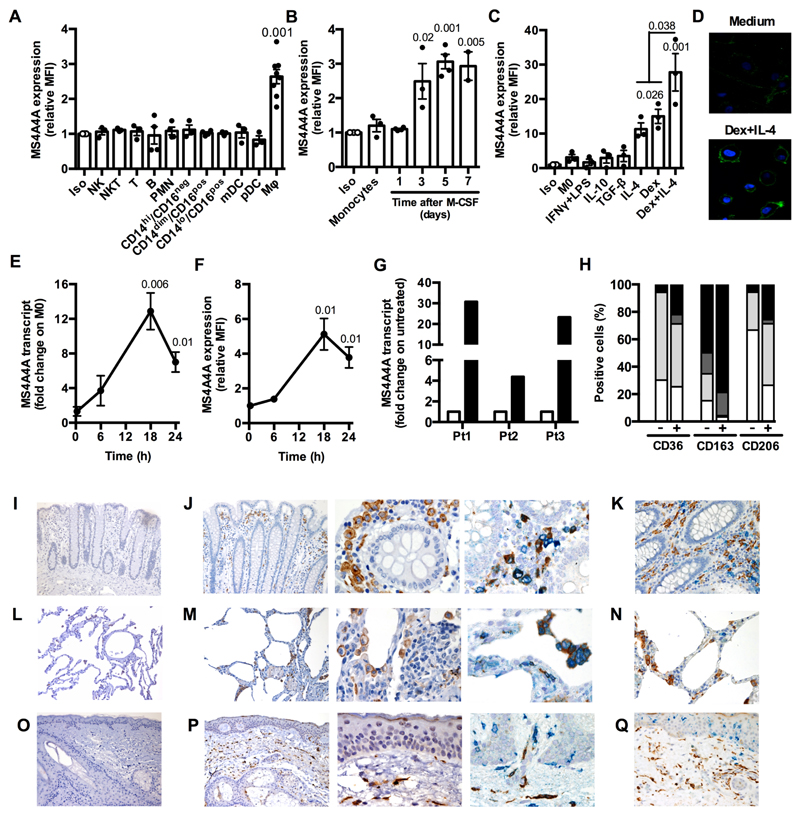
The researchers discovered that MS4A4A is expressed selectively by cells of the monocyte-macrophage lineage and is induced during monocyte-to-macrophage differentiation, but not in dendritic cells. Additionally, M2 or M2-like signals, including IL-4 and dexamethasone, upregulate MS4A4A in macrophages. In vivo, MS4A4A is expressed by human tissue resident macrophages, macrophages infiltrating the inflamed synovium in rheumatoid arthritis patients, and tumor-associated macrophages, and is induced in monocytes in patients treated with methylprednisolone.
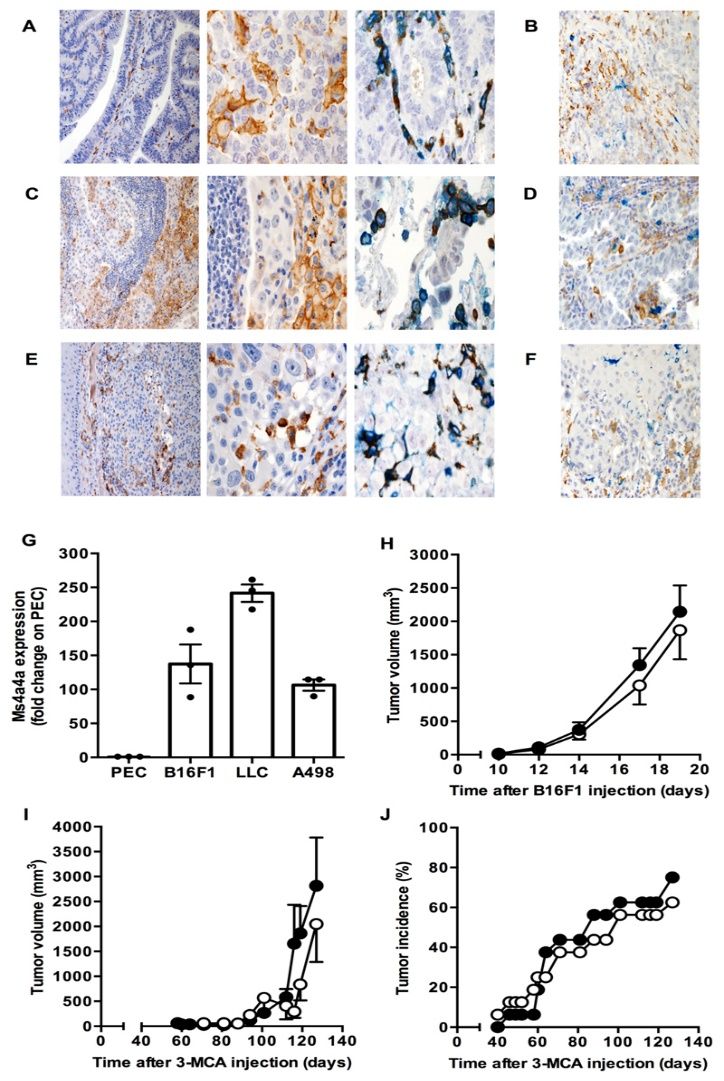
When macrophages engage with Zymosan, an agonist of the β-glucan receptor Dectin-1, MS4A4A colocalizes with Dectin-1 in the lipid rafts. The absence of MS4A4A leads to an impairment in the activation of the Syk pathway downstream Dectin-1, resulting in reduced production of cytokines and reactive oxygen species. It is important to note that MS4A4A deficiency in macrophages has no impact on primary tumor growth, but it does compromise Dectin-1-driven NK cell-mediated resistance to metastasis.
To test the role of MS4A4A in cancer metastasis, the researchers utilized a mouse model of mesenchymal carcinogenesis and transplanted tumor growth. They found that macrophage-selective genetic inactivation of Ms4a4a had no impact on primary tumor growth. Conversely, Ms4a4a-deficient macrophages were impaired in their ability to support Dectin-1-dependent cross-talk with NK cells, which resulted in uncontrolled metastatic spread. These findings shed new light on the critical role that MS4A4A plays in activating NK cells, and highlight the potential of MS4A4A as a therapeutic target for cancer immunotherapy.
The identification of MS4A4A as a key regulator of tumor-associated macrophages represents an important step forward in cancer immunotherapy. By targeting MS4A4A, researchers were able to reshape the tumor immune microenvironment to be more immunostimulatory, enhancing the efficacy of immune checkpoint inhibitors. The studies provide critical insights into how modulating macrophages can unleash the potential of the immune system against cancer.

References:
1.Li Y, Shen Z, Chai Z, et al. Targeting MS4A4A on tumour-associated macrophages restores CD8+ T-cell-mediated antitumour immunity, Gut, 2023;0:1–14.
2.Mattiola, I., Tomay, F., De Pizzol, M. et al. The macrophage tetraspan MS4A4A enhances dectin-1-dependent NK cell–mediated resistance to metastasis. Nat Immunol 20, 1012–1022 (2019).