Rhythm Pharmaceuticals Initiates Phase 2 Trial for Oral MC4R Agonist LB54640 in Hypothalamic Obesity Patients
Rhythm Pharmaceuticals, Inc., a biopharmaceutical company actively engaged in commercial operations and dedicated to improving the lives of patients and families affected by rare neuroendocrine disorders, has reported that initial patients have received doses in its Phase 2 clinical study. This study is assessing the efficacy of LB54640, an experimental oral melanocortin-4 receptor (MC4R) agonist, in the treatment of hypothalamic obesity.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"Drawing from LG Chem's extensive preclinical work and promising outcomes from a Phase 1 trial, we view LB54640 as a potential effective oral therapy for MC4R pathway diseases without causing hyperpigmentation," stated David Meeker, M.D., Chair, Chief Executive Officer and President of Rhythm Pharmaceuticals.
"We are eager to move forward with this molecule, aiming to provide a comprehensive range of treatment options for patients dealing with hyperphagia and severe obesity, ensuring they receive the most suitable therapy," added David Meeker.
The Phase 2 trial is designed as a randomized, placebo-controlled, double-blind study to evaluate the safety, weight loss, appetite suppression, and quality of life impacts of LB54640 in individuals aged 12 and above with hypothalamic obesity. Participants will receive a daily oral dose of either LB54640 or placebo for a duration of 14 weeks. The primary objective is to measure the change in body mass index from baseline after 14 weeks of treatment, with the possibility for patients to remain on therapy for up to 52 weeks.
Rhythm entered into a worldwide licensing agreement with LG Chem, Ltd. for LB54640 in January 2024. During a Phase 1 study involving healthy overweight adults, LB54640 showed dose-related weight loss and had a positive safety profile, with no alterations in blood pressure or heart rate and no signs of hyperpigmentation.
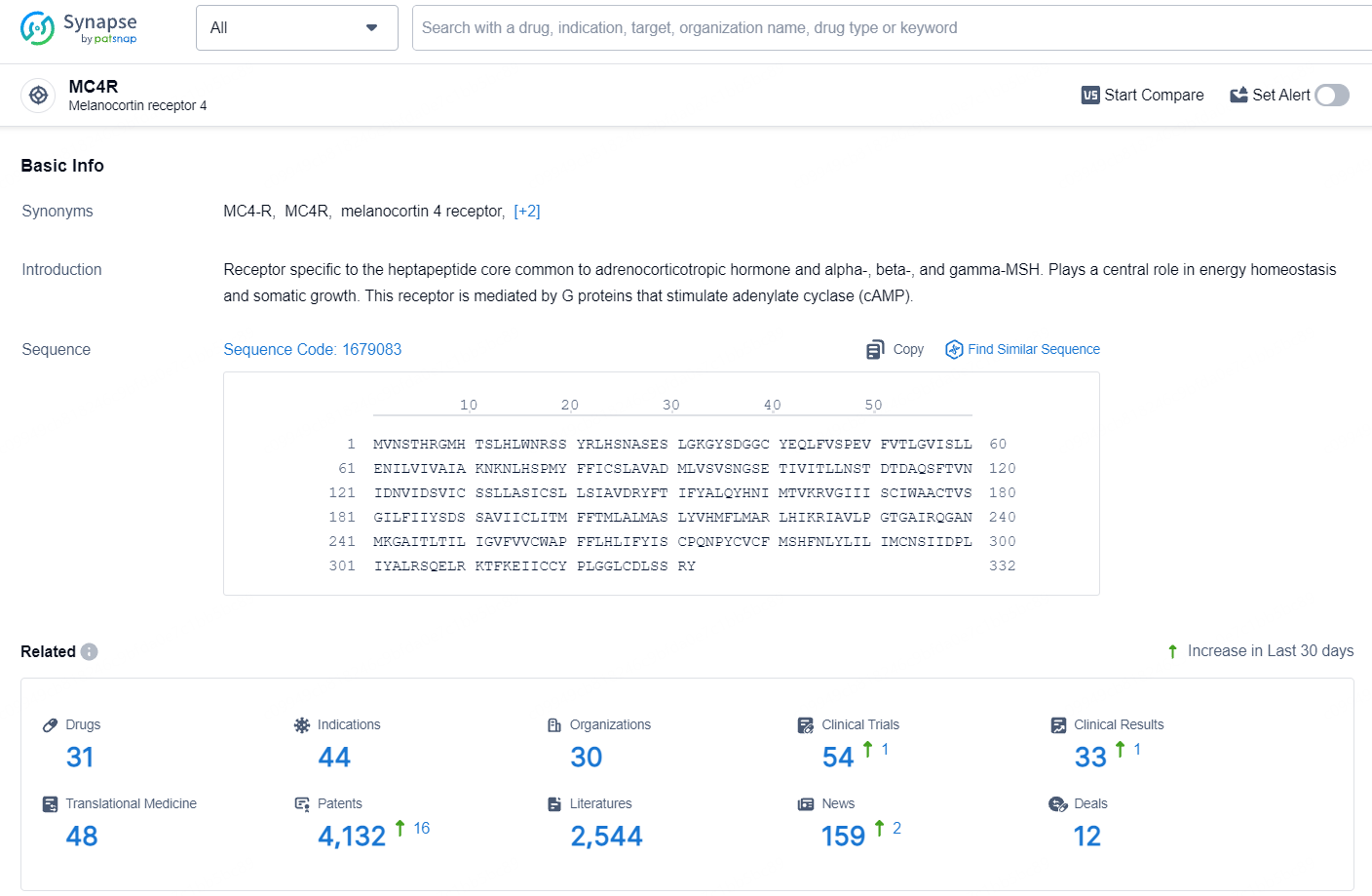
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of July 30, 2024, there are 31 investigational drugs for the MC4R target, including 44 indications, 30 R&D institutions involved, with related clinical trials reaching 54, and as many as 4132 patents.
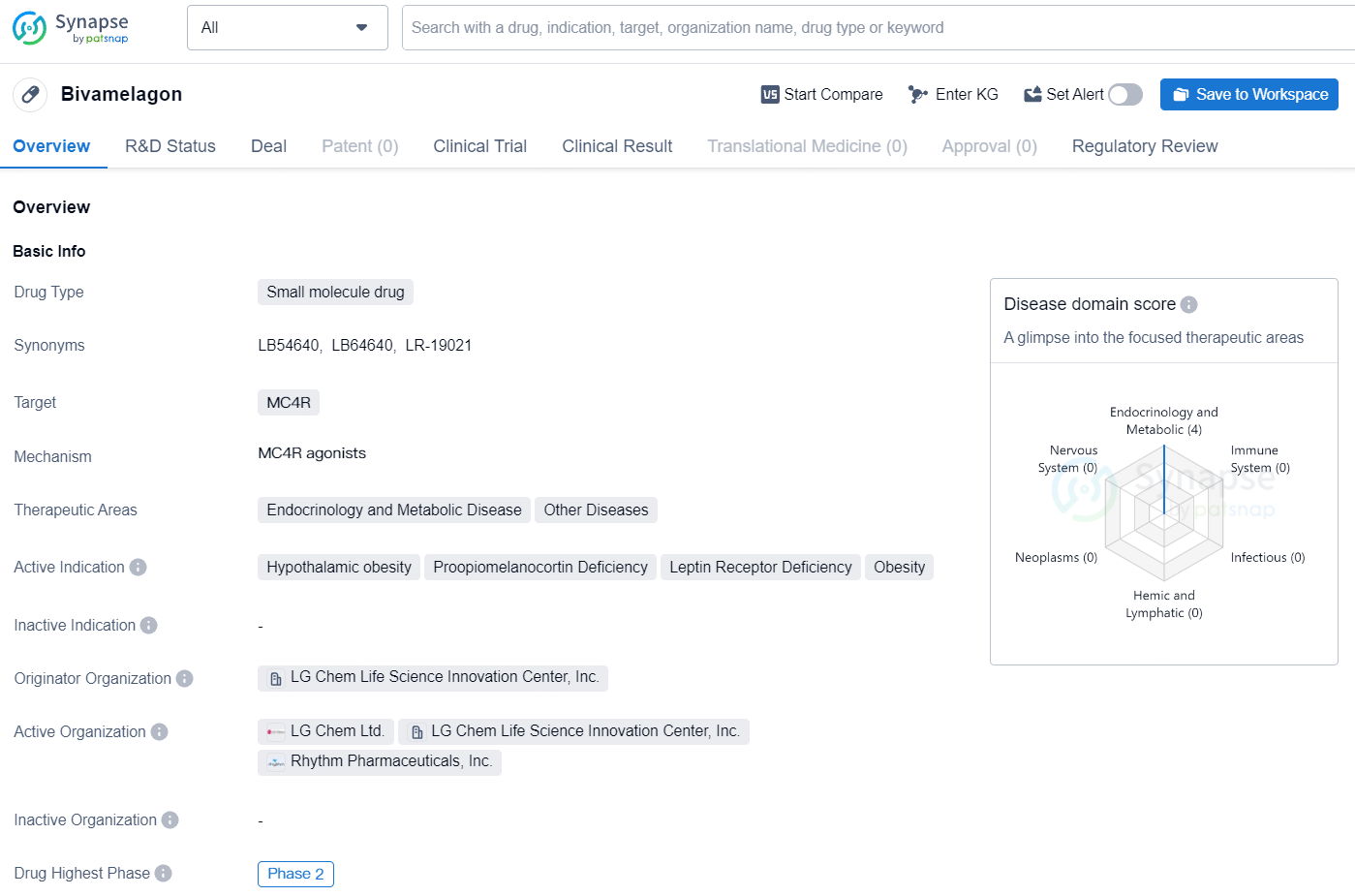
Bivamelagon is a small molecule drug with a specific focus on addressing rare diseases associated with MC4R dysregulation. Its advancement to Phase 2 underscores its potential as a treatment option for conditions such as hypothalamic obesity, proopiomelanocortin deficiency, leptin receptor deficiency, and obesity, offering hope to patients facing these challenging medical situations.