Roche's PD-L1 monoclonal antibody, Atezolizumab subcutaneous formulation, has received approval for marketing by the UK's MHRA
Recently, Roche announced that its PD-L1 targeted antibody, Tecentriq(atezolizumab), has been approved for marketing in the UK by the Medicines and Healthcare products Regulatory Agency (MHRA) as a subcutaneous formulation. The Tecentriq subcutaneous formulation has been approved in the UK for all indications previously approved for the intravenous formulation of Tecentriq, which includes certain types of lung, bladder, breast and liver cancers. Compared to an intravenous infusion which takes 30-60 minutes, a subcutaneous injection of Tecentriq typically only takes about 7 minutes.
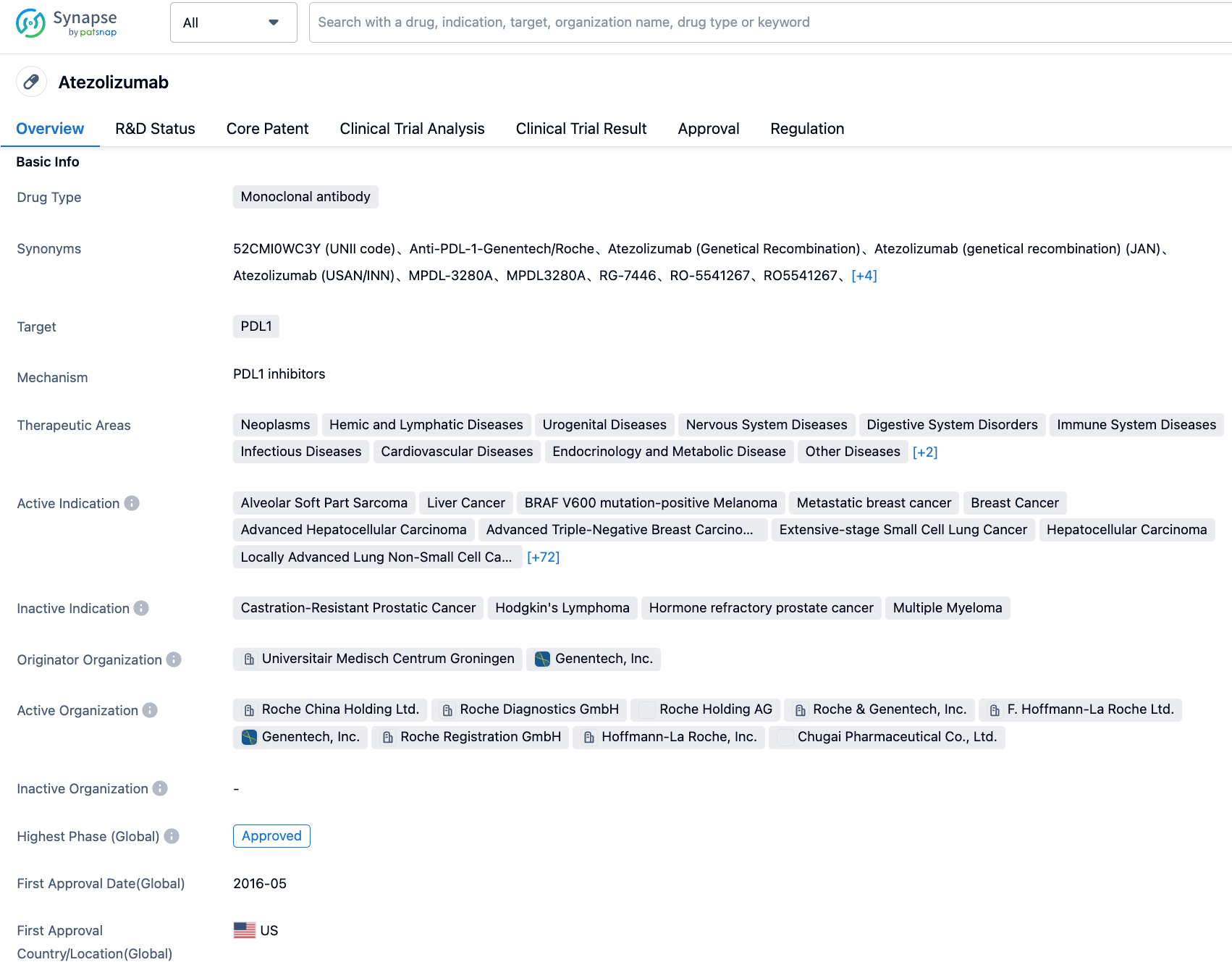
Atezolizumab is a monoclonal antibody developed by Roche to target PD-L1. It works by blocking the interaction between PD-L1 expressed on tumor cells and the PD-1 and B7.1 receptors on immune cells, thereby activating T cells. At present, atezolizumab has been approved globally for the treatment of several types of cancers including non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC), hepatocellular carcinoma, urothelial carcinoma, PD-L1-positive metastatic triple-negative breast cancer, and advanced melanoma with BRAF V600 mutations.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The subcutaneous formulation of atezolizumab combines the monoclonal antibody atezolizumab with Halozyme Therapeutics' Enhanze drug delivery technology. The Enhanze drug delivery technology is based on a proprietary recombinant human hyaluronidase PH20 (rHuPH20), an enzyme that temporarily degrades hyaluronic acid in the human body, allowing the Tecentriq subcutaneous formulation to disperse and be absorbed into the bloodstream quickly.
On December 1, 2022, Roche announced new data from the Phase III IMscin001 study of the PD-L1 antibody atezolizumab (Tecentriq) subcutaneous formulation: in patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) who have previously been treated, subcutaneous injection of atezolizumab has comparable exposure (molecular level in the blood) and similar efficacy and safety compared to standard intravenous infusion. Roche has submitted data from the IMscin001 study to regulatory authorities worldwide and is seeking approval for the subcutaneous formulation of atezolizumab for all approved indications of the intravenous formulation. The subcutaneous formulation of atezolizumab shows similar efficacy to the intravenous infusion group and is consistent with the known conditions of the intravenous formulation.
Specifically, the median PFS for the subcutaneous injection and intravenous infusion groups were 2.8 months and 2.9 months respectively, and their ORRs were 11.8% and 9.7% respectively. The safety of the subcutaneous formulation is also consistent with the intravenous formulation.
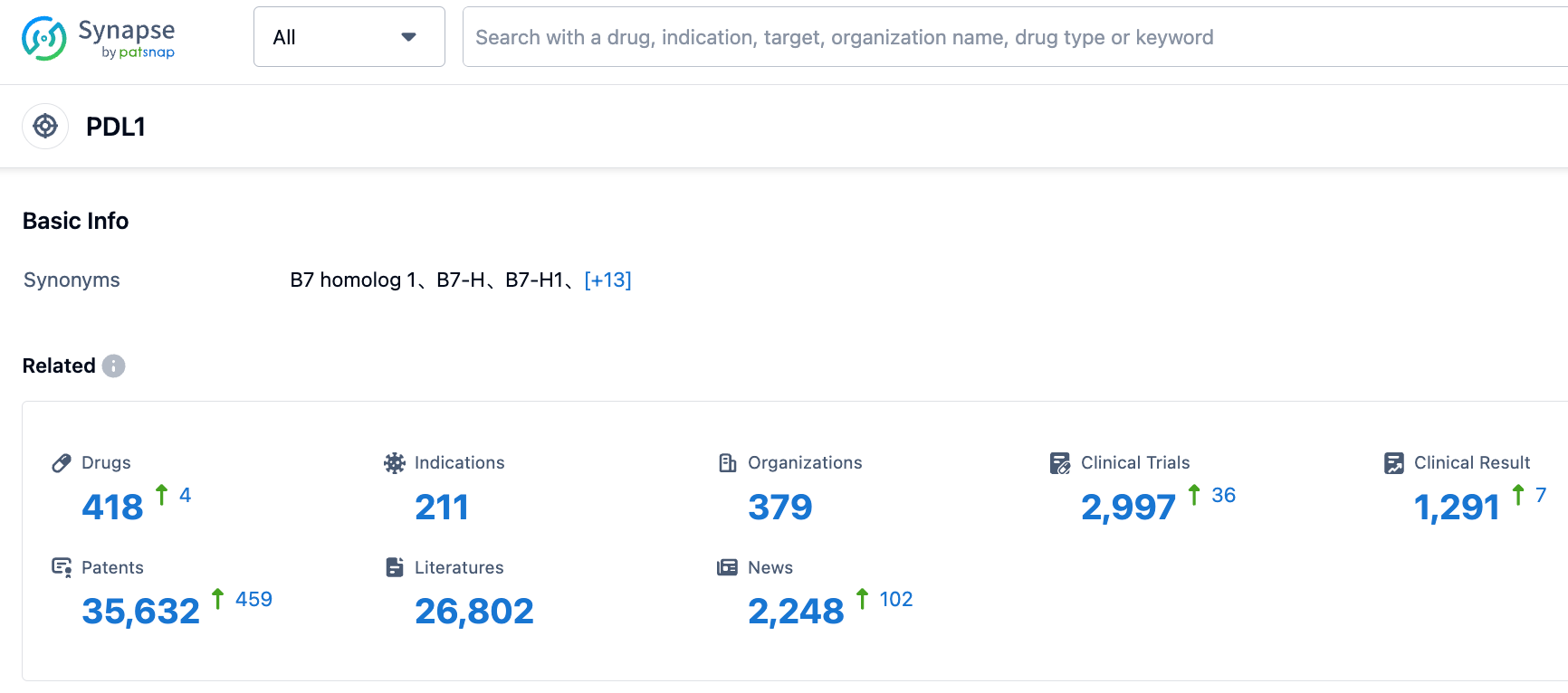
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to information disclosed by the Synapse database, as of September 1, 2023, there are 418 drugs under research targeting PD-L1, with 211 indications, 379 research institutions involved, 2,996 related clinical trials, and as many as 35,653 patents... The subcutaneous formulation of Atezolizumab is Roche's fourth subcutaneous cancer treatment. Multiple oncology studies have shown that, compared to intravenous administration, most cancer patients generally prefer subcutaneous administration because it is less uncomfortable, easier to administer, and the treatment duration is shorter. The approval of this new mode of administration is expected to boost the sales of Atezolizumab.