Roche's PiaSky receives EU approval as the first monthly subcutaneous therapy for PNH patients
Roche has announced that the European Commission has granted approval for PiaSky® (crovalimab), an innovative recycling monoclonal antibody that targets the complement protein C5. This approval covers use in adults and adolescents (aged 12 years and older with a minimum weight of 40 kg) who suffer from paroxysmal nocturnal haemoglobinuria (PNH) and are either naïve to C5 inhibitors or have previously undergone treatment with them. PNH is a rare, life-threatening haematological disorder characterized by the destruction of red blood cells by the complement system, a component of the innate immune defence, leading to symptoms such as anaemia, fatigue, and thrombosis, and possibly resulting in renal disease.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Individuals with PNH frequently endure continual, life-long intravenous infusions that necessitate time-intensive clinic visits. This dependency significantly impacts their daily lives, as well as those of their caregivers and families, by making treatment demands a central focus," said Prof. Alexander Röth, M.D., the Head of Classical Haematology and Haemostasis at the West German Cancer Centre, University Hospital Essen, Germany. "Treatment options with greater flexibility, such as PiaSky, which are equally effective but less frequent and can be administered more swiftly at home, are crucial for providing individuals with PNH more autonomy over their treatment and fostering greater independence."
PiaSky represents the first monthly subcutaneous (SC) treatment for PNH within the European Union, with a provision for self-administration after suitable training. This treatment serves as an alternative to current C5 inhibitors that require regular intravenous infusions, offering potential to alleviate the treatment burden and life disruptions experienced by both PNH patients and their caregivers.
"The approval of PiaSky introduces a new alternative in PNH treatment, combining the disease management achieved by C5 inhibition with advanced recycling technology, facilitating monthly subcutaneous administration," said Levi Garraway, M.D., Ph.D., Roche’s Chief Medical Officer and Head of Global Product Development. "We are excited to offer this novel treatment to PNH patients in Europe and hope it will ease the treatment burdens many face."
C5 inhibitors, which function by blocking a part of the complement system cascade, have proven effective in managing PNH. PiaSky was specifically developed to meet the needs of PNH patients and address certain challenges associated with existing treatments. It improves complement inhibition through an innovative recycling technology, allowing monthly SC administration by enabling the medicine to bind and inhibit the C5 protein repeatedly, thus maintaining longer efficacy in the body with a reduced volume of medicine.
This approval is founded on data from the Phase III COMMODORE 2 study, which involved PNH patients who had not undergone previous C5 inhibitor treatment. The study indicated that monthly SC injections of PiaSky effectively controlled the disease and were well-tolerated. PiaSky was found to be non-inferior, with comparable safety to eculizumab, an existing standard C5 inhibitor given biweekly intravenously. Adverse event rates were similar between those treated with PiaSky and those receiving eculizumab. Additional supportive data came from two other Phase III studies: the COMMODORE 1 study, which included PNH patients transitioning from other C5 inhibitors, and the COMMODORE 3 study, involving patients in China new to C5 inhibitor treatment.
PiaSky is the first monthly SC treatment for PNH approved in various regions globally, including the US and Japan, based on findings from the COMMODORE studies. It is currently being researched further in a comprehensive clinical development program, encompassing five Phase III studies and three earlier-phase studies focused on complement-mediated diseases, such as PNH, atypical haemolytic uremic syndrome, and sickle cell disease.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
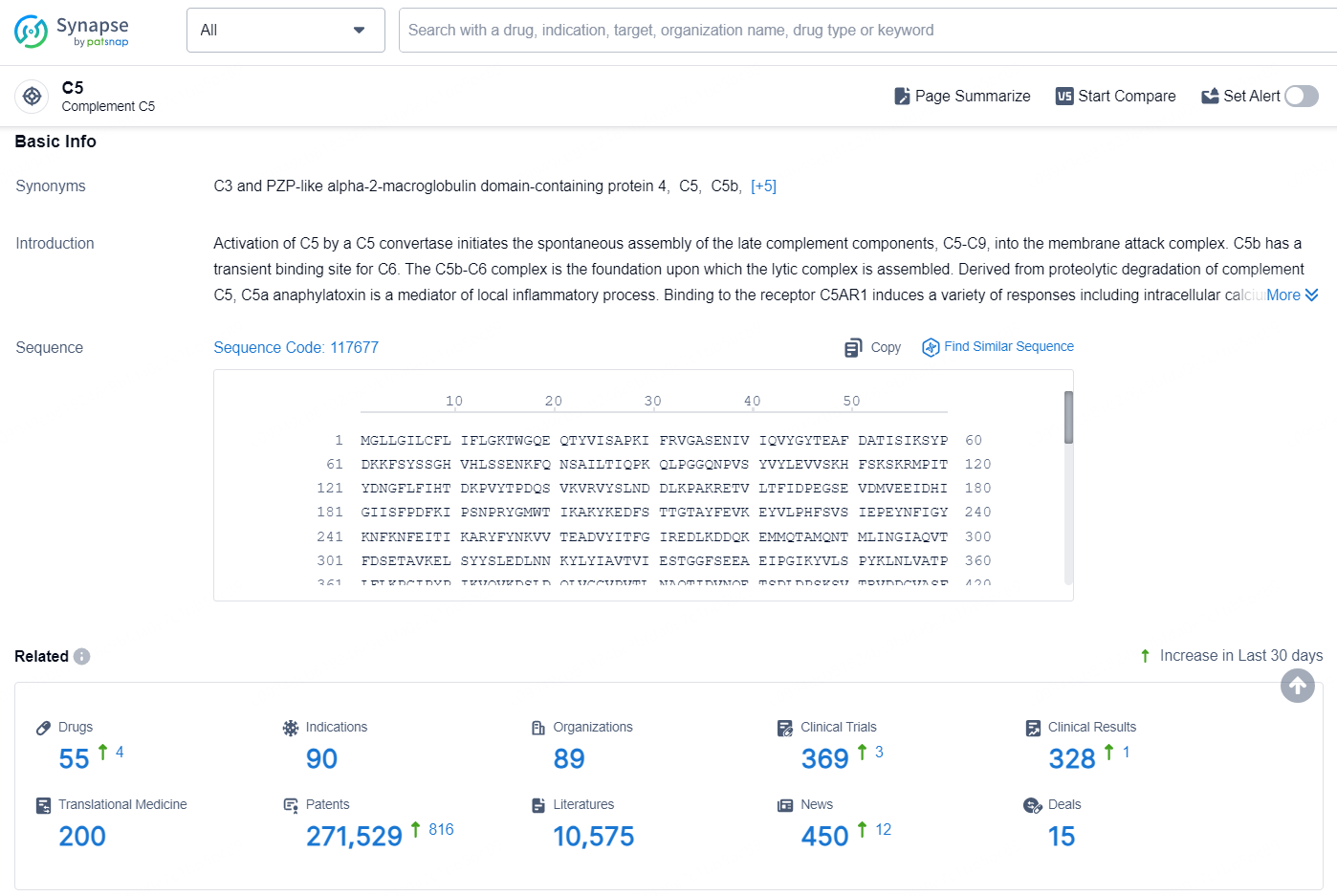
According to the data provided by the Synapse Database, As of August 29, 2024, there are 55 investigational drugs for the C5 target, including 90 indications, 89 R&D institutions involved, with related clinical trials reaching369, and as many as 271529 patents.
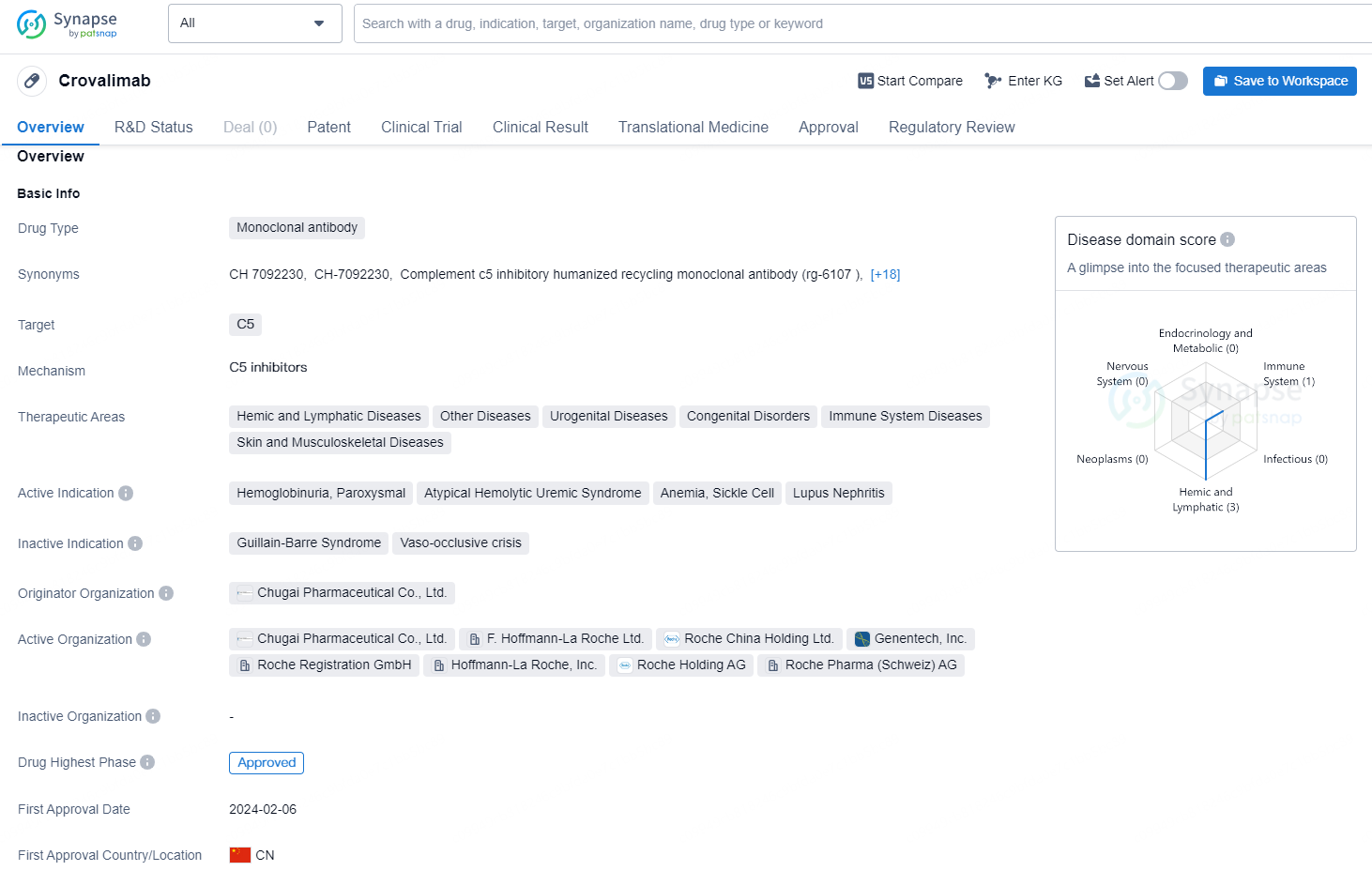
Crovalimab is a monoclonal antibody drug that targets the C5 protein. It is utilized in the treatment of various therapeutic areas, including Hemic and Lymphatic Diseases, Other Diseases, Urogenital Diseases, Congenital Disorders, Immune System Diseases, and Skin and Musculoskeletal Diseases. The drug is indicated for specific conditions such as Hemoglobinuria, Paroxysmal, Atypical Hemolytic Uremic Syndrome, Anemia (Sickle Cell), and Lupus Nephritis.