Scholar Rock Reveals SRK-439 Data: Preserves Lean Mass, Reduces Fat Mass Rebound Post-GLP-1 Agonist
Scholar Rock, an advanced biopharmaceutical company dedicated to developing novel therapies for spinal muscular atrophy, cardiometabolic diseases, and other significant conditions influenced by protein growth factors, has reported the initial dosing of participants in the Phase 2 EMBRAZE proof-of-concept study. This trial aims to evaluate the safety and effectiveness of apitegromab, a highly selective experimental myostatin inhibitor, in maintaining lean muscle mass in individuals with obesity who are receiving background treatment with a GLP-1 receptor agonist (GLP-1 RA).
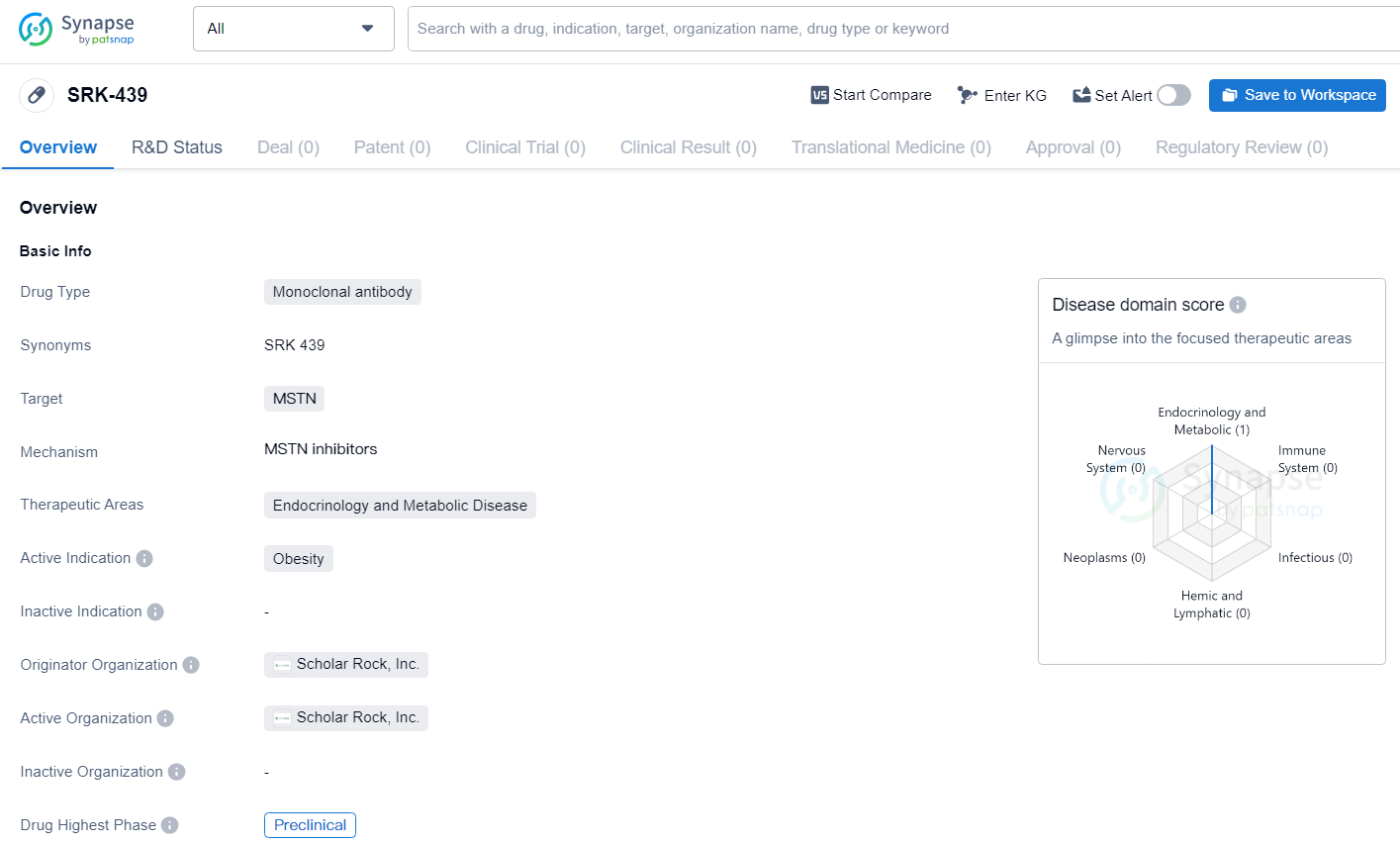
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The study will also assess apitegromab’s impact on the sustained weight loss after discontinuation of GLP-1 RA therapy. Findings from this research will aid in the progression of SRK-439, a new investigational selective myostatin inhibitor designed for treating cardiometabolic conditions, such as obesity.
Additionally, the Company unveiled new preclinical findings demonstrating SRK-439’s potential to enhance lean mass and promote a favorable body composition after GLP-1 RA therapy cessation. These findings were presented by Dr. Melissa Fulham of Scholar Rock at the American Diabetes Association’s 84th Scientific Sessions held on June 23rd in Orlando, Florida.
“These fresh preclinical findings provide strong evidence that SRK-439 helped to preserve lean muscle during GLP-1 RA-driven weight loss and reduced fat mass rebound following semaglutide discontinuation,” stated Dr. Mo Qatanani, Chief Scientific Officer at Scholar Rock. “Mice that received SRK-439 treatment showcased significantly more lean mass at the conclusion of the semaglutide withdrawal phase. These promising data continue to highlight the unique profile of SRK-439 and its potential for contributing to healthier weight management and prolonged metabolic advantages during and after GLP-1 RA therapy.”
SRK-439 is a novel, investigational myostatin inhibitor in the preclinical stage, which selectively binds to pro- and latent myostatin with high affinity. It is initially being developed for the treatment of cardiometabolic disorders, including obesity. Based on preclinical evidence, SRK-439 might support healthier weight management by maintaining lean mass during weight loss. The efficacy and safety of SRK-439 are not yet established, and it has not been approved for any use by the FDA or any other regulatory authority.
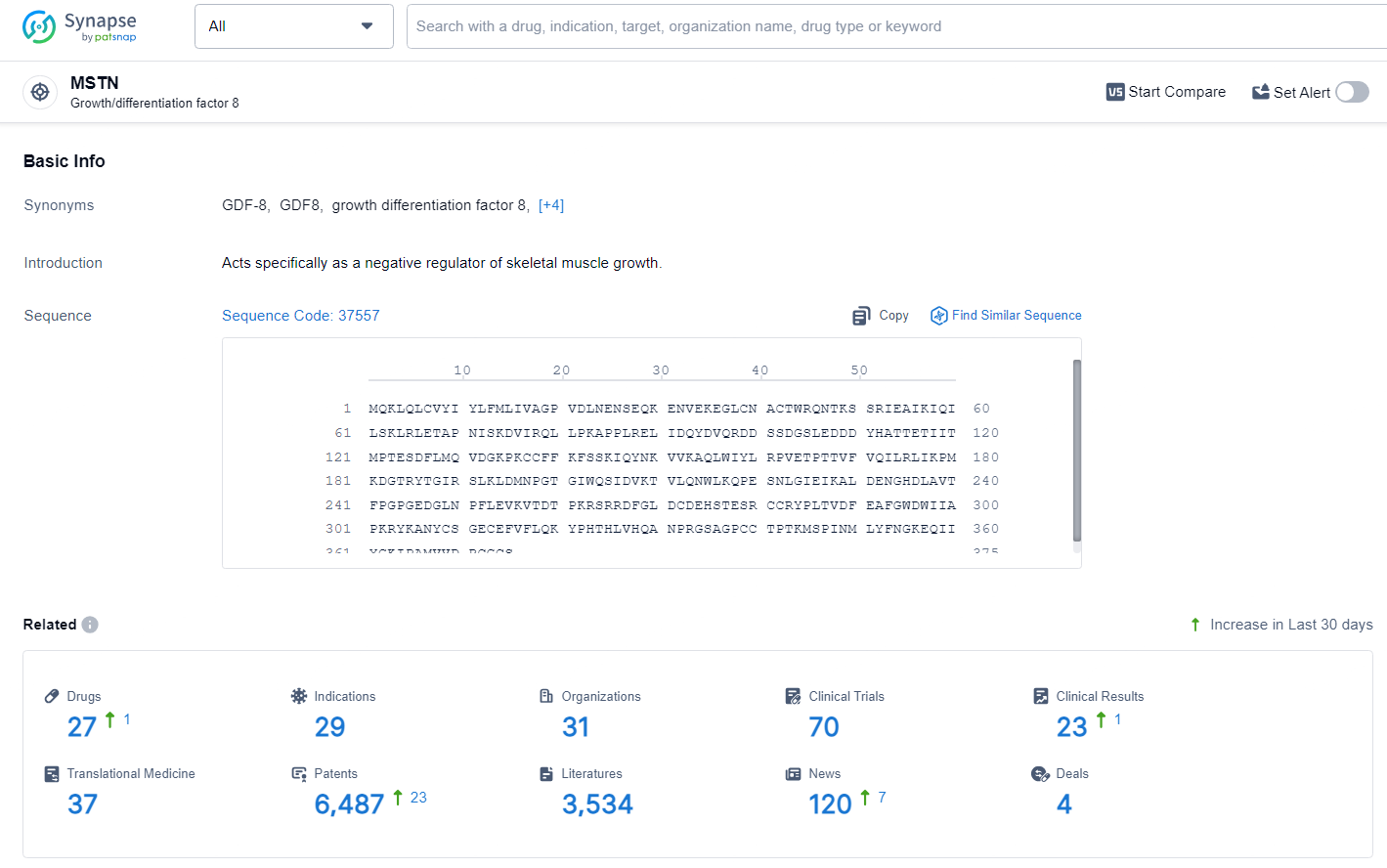
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 27, 2024, there are 27 investigational drugs for the MSTN target, including 29 indications, 31 R&D institutions involved, with related clinical trials reaching 70, and as many as 6487 patents.
SRK-439 targets MSTN and is intended for use in treating obesity within the therapeutic areas of endocrinology and metabolic disease. With the drug currently in the preclinical phase, further research and development efforts are likely underway to evaluate its potential as a treatment for obesity.