Shaperon Begins Phase 2 US Clinical Trial Enrollment for Nugel Eczema Treatment
Shaperon, Inc., a company at the forefront of biotechnological advancements, has publicized the initiation of their Phase 2 clinical study, where the inaugural participant in the trial has been registered. This participant will be administered Nugel, Shaperon's cutting-edge therapeutic drug designed to combat Atopic Dermatitis. This key development signifies the commencement of an important phase for Shaperon as they strive to enhance therapeutic options for patients around the world.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
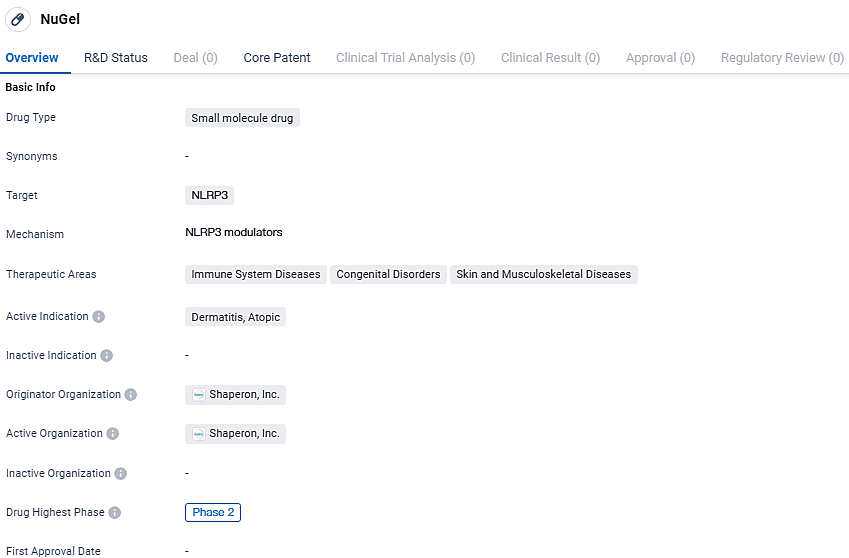
Having obtained green light from the FDA in September 2023 for the commencement of global Phase 2 Clinical Trials, Shaperon is thoroughly assessing Nugel’s ability to enhance the Eczema Area Severity Index among a varied group of 210 individuals suffering from light to moderate atopic dermatitis. The study employs a strict double-blind process, compared against a placebo, and is projected to wrap up in the early part of 2026.
Considering the market analysis, a vigorous expansion of the market for atopic dermatitis treatments is predicted, with an 8.7% increase leading to an estimated value of $19.1 billion by the year 2030. Currently, steroid-based therapies and other medications approved by the FDA are standard treatments, but there remains an urgent demand for innovative drugs that bypass the negative side effects and limitations of existing options.
Nugel is at the forefront of a new era in atopic dermatitis management, being the first medication to modulate the activity of ‘GPCR19’ for controlling inflammation. This drug’s distinctive mode of action is limited to immune-regulatory cells and is designed to mitigate inflammation with reduced adverse reactions. The potential of Nugel extends beyond atopic dermatitis, showing promise for a wider range of dermatological disorders and signaling a significant shift in the future of global medical treatments.
Shaperon’s CEO, Dr. Seung-Yong Seong, stands firm on the promise to propel Nugel into the international market and to ensure the successful exchange of technology throughout the ongoing Phase 2 trials. Shaperon is employing forward-thinking methods, including a smartwatch-integrated AI for tracking and managing medication, to enhance the care of patients and the accuracy of clinical data.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
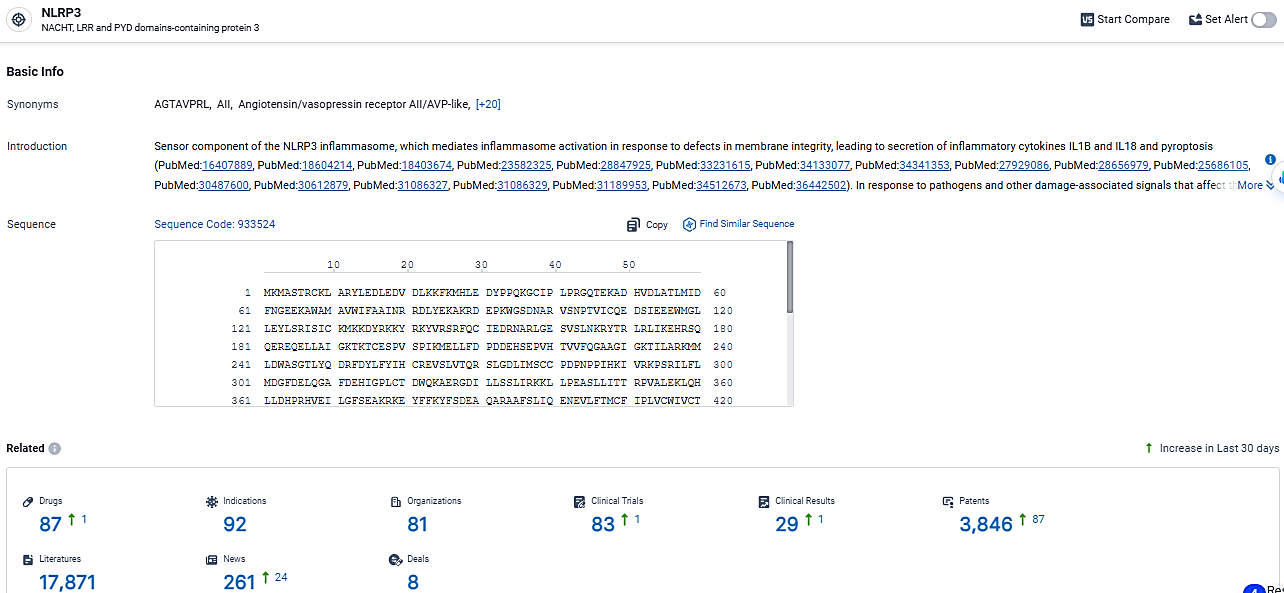
According to the data provided by the Synapse Database, As of March 19 2024, there are 87 investigational drugs for the NLRP3 target, including 92 indications, 81 R&D institutions involved, with related clinical trials reaching 83, and as many as 3846 patents.
NuGel is a small molecule drug being developed by Shaperon, Inc. that targets NLRP3. It is being investigated for its potential in treating immune system diseases, congenital disorders, and skin and musculoskeletal diseases, with a specific focus on atopic dermatitis. Currently in Phase 2 of clinical development, NuGel shows promise in modulating the immune response and addressing the unmet needs in these therapeutic areas.