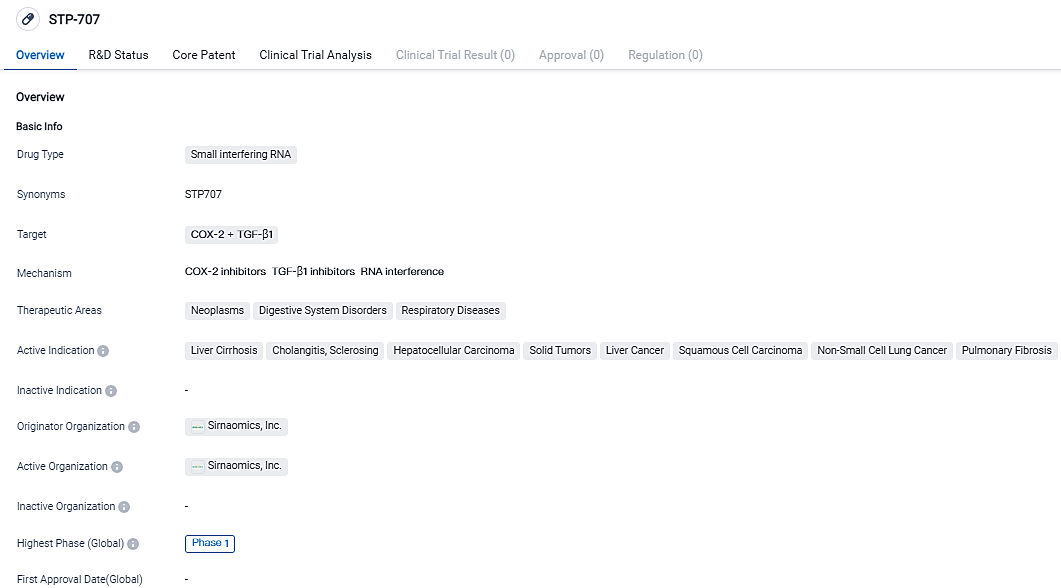
Sirnaomics Announces Successful Phase I clinical trial for STP707, a RNAi therapeutic for addressing multiple solid tumors
Sirnaomics Ltd., a major participant in the field of biopharmaceuticals specializing in the exploration and progress of therapeutics utilizing RNAi, proclaimed that the team has thoroughly dispatched all dosage regimens for their STP707 Phase I examination designed for treating numerous solid tumors in patients grappling with profound variety of advanced cancers, unresponsive to multiple courses of alternative oncology therapies. A striking 74% of assessable patients showcased a premier response of steady disease also, several patients displayed a decrease in tumor load as per the imperative Response Evaluation Criteria in Solid Tumors.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
CEO, President, and Chairman of Sirnaomics, Dr. Patrick Lu, announced, "For the first time in the RNAi cancer therapeutics arena, a Phase I study has portrayed an extremely optimistic clinical possibility for metastasized tumors. We noticed patients attaining steady disease condition with a handful experiencing a shrinkage in tumor capacity after STP707 treatment." He added that the findings from this basket study urge the pursuit of STP707 as a potential solitary medication or as a joint cure with immune check point inhibitor drugs.
The basket study involved the participation of 50 patients affected by advanced solid tumors inclusive of pancreatic cancer, liver cancer, colon cancer, ovarian cancer, and melanoma. All these patients had demonstrated progressive disease post previous rounds of treatment using market available oncology pharmaceuticals. Preliminary efficacy observations indicated that 74% of assessable patients demonstrated a premier response of stable disease with several patients showing a decrease in tumor load per RECIST.
The clinical examination was a collaborative effort of numerous esteemed cancer institutions in the US including Mayo Clinic Oncology, Yale Cancer Center, Next Oncology, Emory Cancer Center, and University of Southern California/Hoag. The multicentre, label-free, dose amplification study was aimed at evaluating the safety, tolerability, and anti-cancer activity of STP707. The entrants received doses ranging from 3mg to 48mg via intravenous administration once every week, making up four doses over a period of 28 days. The cure will continue until the patients display progressive disease.
Sirnaomics' Chief Medical Officer, Dr. Michael Molyneaux, remarked, "STP707 has demonstrated a strong safety profile compared to other emerging oncology therapeutics, with 74% of assessable patients displaying a premier response of stable disease and a few patients showing a reduction in tumor load per RECIST." He emphasized that the group of patients in the study were previous recipients of multiple treatments, all of which proved unsuccessful. The promising safety profile combined with the response duration encourages the team to proceed with the study.
Additional details of the clinical trial are available under the identifier NCT05037149 at clinicaltrials.gov. STP707 consists of two siRNA oligonucleotides, focused on TGF-β1 + COX-2 mRNA respectively, designed in nanoparticles with a Histidine-Lysine Co-Polymer (HKP+H) peptide acting as the carrier. Its carrier peptide is distinct from that used in Sirnaomics' STP705 product. Each separate siRNA was verified to restrict the expression of their objective mRNAs, and their combination results in a synergistic effect reducing pro-inflammatory factors. When delivered through IV, STP707 resulted in the shutdown of TGF-β1 and COX-2 gene expressions in several organs, including the liver, lungs and xenograft tumor in preclinical studies.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
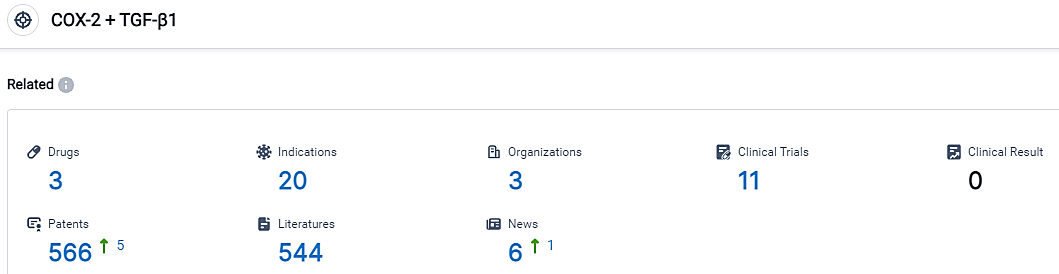
According to the information disclosed by the Synapse Database, as of September 1, 2023, there are 3 investigational drugs for the TGF-β1 + COX-2 target, including 20 applicable indications, 3 R&D institutions involved, with related clinical trials reaching 11, and as many as 566 patents.
Sirnaomics is an innovator within the biopharmaceutical field focusing on RNA therapeutics, with product contenders in both pre-clinical and clinical stages. This company is aimed at inventing groundbreaking medicines for conditions that hold considerable medical necessities and market potential. Sirnaomics is recognized as the initial RNA therapeutics company in the clinical-stage to mark its robust existence in both the United States and Asia. Apartment from this, they have developed proprietary delivery systems consisting of Polypeptide Nanoparticle Formulation and the second-generation GalNAc conjugation. This has resulted in a comprehensive pipeline of potential drug candidates.
Currently, Sirnaomics holds a commanding presence in the field of RNAi therapeutics for oncology, with its clinical programs for STP705 and STP707 registering numerous accomplishments. Furthermore, STP122G is the maiden GalAhead™ technology-based drug contender under clinical development. With the construction of its production facility, Sirnaomics is transitioning from a biotech firm to a full-fledged biopharma corporation.