Market Analysis of Sodium Nitroprusside in the USA: Patent Landscape
Overview
The US market currently has 1 approved drug containing Sodium Nitroprusside for the treatment of hypertension and heart failure. Sodium Nitroprusside is a potent vasodilator that acts rapidly to reduce blood pressure by releasing nitric oxide. The drug has been in clinical use for decades, with the original approval dating back to 1974. Currently, Exela Pharma Sciences LLC holds the approved formulations in the US market. The drug appears to be off-patent in the US with no core patents listed in the FDA Orange Book, suggesting a mature market with potential for generic competition.
Detailed Description
Drug Information
Sodium Nitroprusside was originally developed by F. Hoffmann-La Roche Ltd. and has been approved in the USA since 1974.
| Approval Number | Approval Company | Approval Date | Dosage Form | Specification | Administration Route | Indication | Approval Status |
|---|---|---|---|---|---|---|---|
| 017546 | Hoffmann-La Roche, Inc. | 1974-05-10 | Injection | 50MG/VIAL | Injection | Hypertension, Heart Failure | Withdrawn |
| 018450 | AbbVie, Inc. | 1981-09-08 | Injection | 50MG/VIAL | Injection | - | Withdrawn |
| 018581 | BAXTER HEALTHCARE CORP ANESTHESIA AND CRITICAL CARE | 1982-07-28 | Injection | 50MG/VIAL | Injection | - | Withdrawn |
| 209387 | Exela Pharma Sciences LLC | 2017-03-08 | Solution | 50MG/100ML (0.5MG/ML) | Intravenous | Heart Failure, Hypertension | Approved |
| 209387 | Exela Pharma Sciences LLC | 2017-03-08 | Solution | 10MG/50ML (0.2MG/ML) | Intravenous | Heart Failure, Hypertension | Withdrawn |
| 209387 | Exela Pharma Sciences LLC | 2017-03-08 | Solution | 20MG/100ML (0.2MG/ML) | Intravenous | Heart Failure, Hypertension | Approved |
Structure
Patent Barrier Analysis
Registration Patent Analysis
No core patents were found in the FDA Orange Book for Sodium Nitroprusside, indicating that the compound is off-patent in the United States.
Other Patent Barrier Analysis
Although there are no core patents in the US, there are several patents held by various companies globally, primarily focused on manufacturing processes, formulations, and new uses:
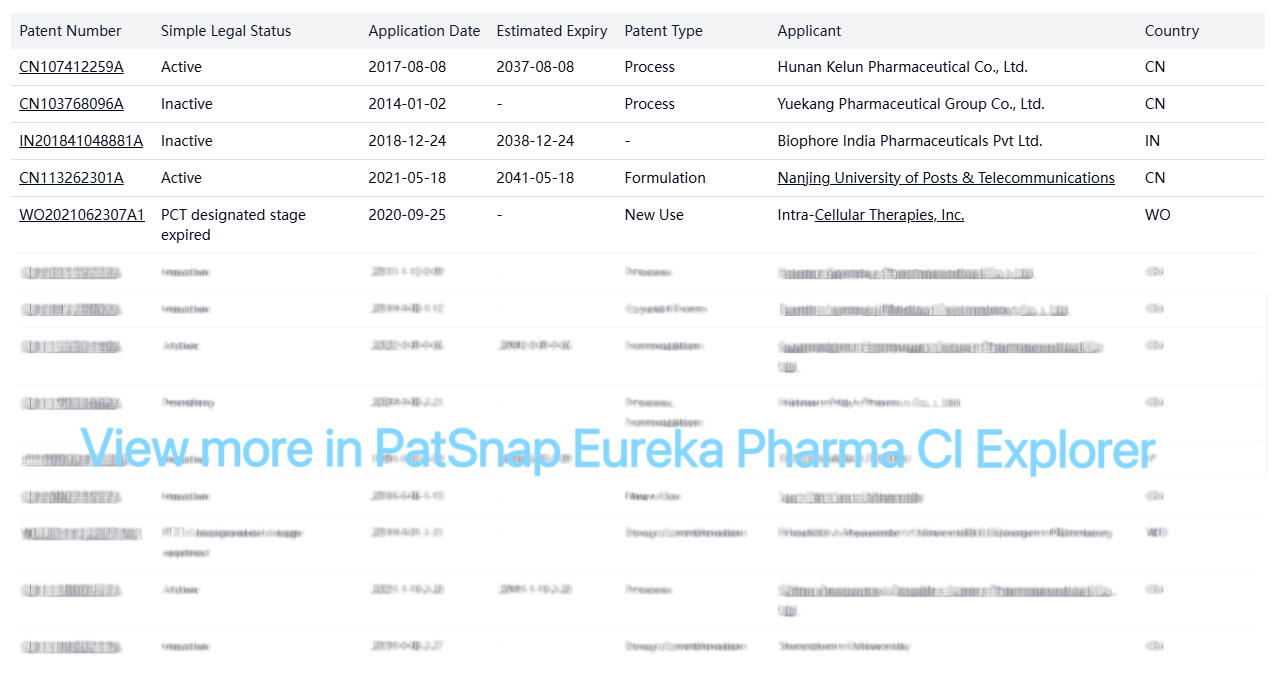
These patents are primarily from Chinese companies and academic institutions, with none directly impacting the US market. They focus on manufacturing processes, formulations, and new uses rather than the core compound itself.
Clinical Results
Based on FDA label clinical insights:
Pharmacodynamic and Mechanistic Studies:
- Sodium Nitroprusside works by interacting with oxyhemoglobin to produce nitric oxide (NO), which activates guanylate cyclase in vascular smooth muscle, increasing cyclic GMP levels, decreasing intracellular calcium, and causing vasodilation.
- The drug has a rapid onset and offset of action with an extremely short circulatory half-life (about 2 minutes).
Controlled Clinical Trials:
- Baseline-controlled clinical trials have confirmed that Sodium Nitroprusside produces a prompt and predictable reduction in blood pressure in both hypertensive and normotensive patients.
- Studies in patients with acute heart failure showed beneficial effects including decreased peripheral resistance, reduced left ventricular filling pressures, and improvements in cardiac output .
- Surgical application studies demonstrated its capacity to induce controlled hypotension, reducing blood loss during major surgeries.
Pediatric Investigations:
- Specialized trials in pediatric populations (under 17 years of age) have evaluated the efficacy and safety of Sodium Nitroprusside for achieving controlled hypotension.
Adverse Reaction Evaluations:
- Detailed investigations have monitored adverse events including excessive hypotension, cyanide toxicity, and thiocyanate toxicity, leading to warnings and precautionary recommendations for careful monitoring during administration.
Infringement Cases
No patent infringement incidents involving Sodium Nitroprusside were identified in the available references.
Policy and Regulatory Risk Warning
After a comprehensive search, Sodium Nitroprusside has no market exclusivity or data protection period in the United States. The compound has been available for decades, with the original approval dating back to 1974, and all relevant patents appear to have expired.
Market Entry Assessment & Recommendations
Based on the analysis, Sodium Nitroprusside presents a mature market opportunity with minimal patent barriers in the United States:
Generic Competition Opportunity: With no core patents listed in the FDA Orange Book and the original compound being off-patent, there are minimal barriers to generic entry from a patent perspective. Companies looking to enter this market should focus on formulation development and regulatory approval processes.
Manufacturing Process Innovation: Given the numerous process patents globally, companies entering the US market should develop non-infringing manufacturing processes or consider licensing arrangements with process patent holders if planning global production.
Formulation Differentiation: Consider developing improved formulations with enhanced stability, safety profiles, or delivery mechanisms to differentiate from existing products, as the current market offerings are limited to intravenous solutions.
Pricing Strategy: As a mature product with limited patent protection, competitive pricing will be essential for market entry. Consider participating in hospital formulary contracts and government procurement programs.
Niche Applications: Explore potential new indications or specialized applications (such as pediatric formulations or surgical applications) to carve out specific market segments and potentially obtain new regulatory exclusivity periods.
Supply Chain Reliability: Given the critical nature of this medication in emergency and surgical settings, ensuring a stable and reliable supply chain could be a significant competitive advantage, especially in light of drug shortages that have affected this product in the past.
Regulatory Focus: Concentrate on meeting all FDA requirements for Abbreviated New Drug Applications (ANDAs) and consider whether any special requirements apply to this product given its safety profile and narrow therapeutic window.
Market Education: Despite being a well-established drug, continued education of healthcare providers about proper administration and monitoring could help expand appropriate use and market share.
The Sodium Nitroprusside market in the USA represents a stable opportunity for generic manufacturers, with limited patent barriers but requiring careful attention to formulation development, manufacturing processes, and regulatory compliance.
For more scientific and detailed information of Sodium Nitroprusside, try PatSnap Eureka Pharma CI Explorer.