Sparsentan: A Breakthrough in Diverse Therapeutic Areas with Advanced Regulatory Approvals
Sparsentan is a small molecule drug that targets AT1R x ETA and has been approved for use in the treatment of a variety of therapeutic areas, including immune system diseases, urogenital diseases, cardiovascular diseases, congenital disorders, hemic and lymphatic diseases, and skin and musculoskeletal diseases, along with other diseases.
The originator organization of Sparsentan is Bristol Myers Squibb Co., and it has received its first approval in the United States in February 2023. The regulation for the drug includes priority review, accelerated approval, and orphan drug status.
Below, we will use the drug Sparsentan as an example to demonstrate how to quickly obtain information about its chemical structure and patent situation using the Patsnap Chemical.
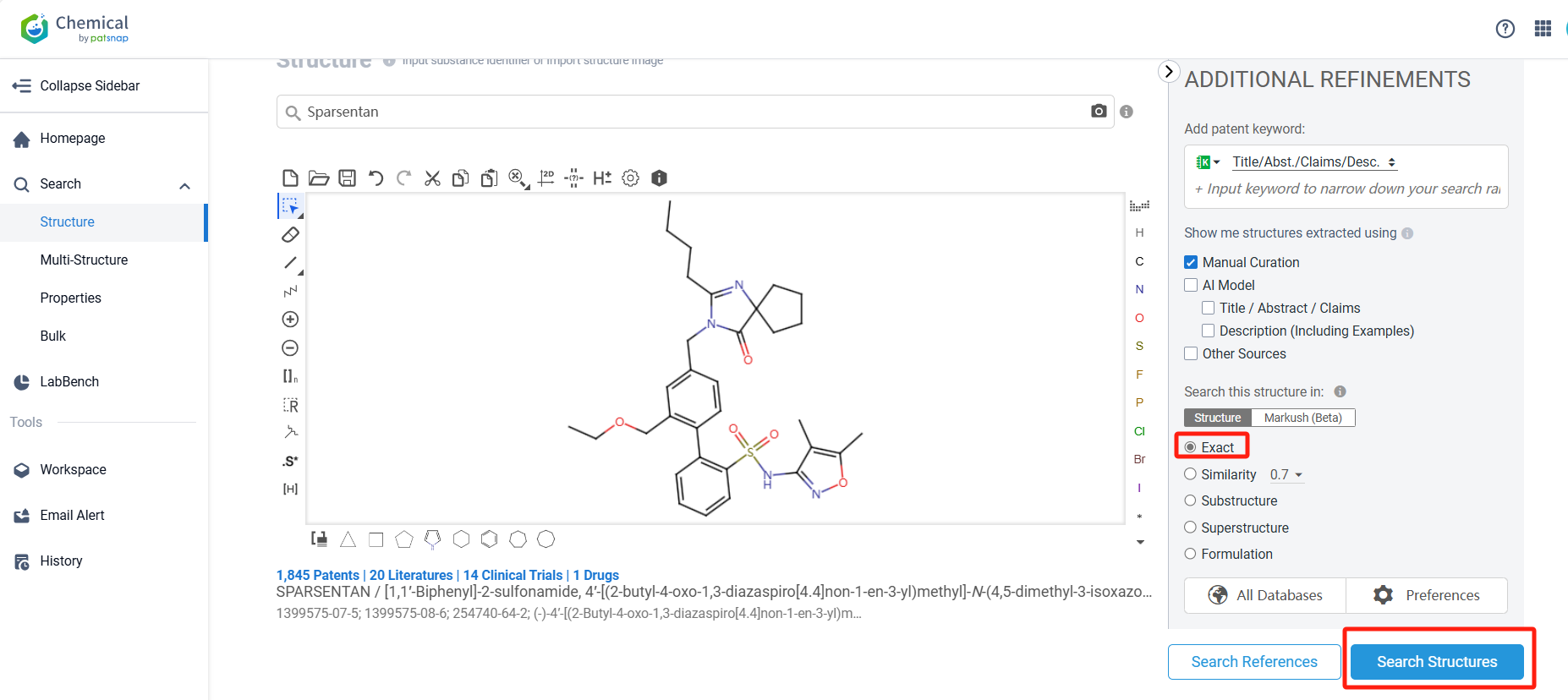
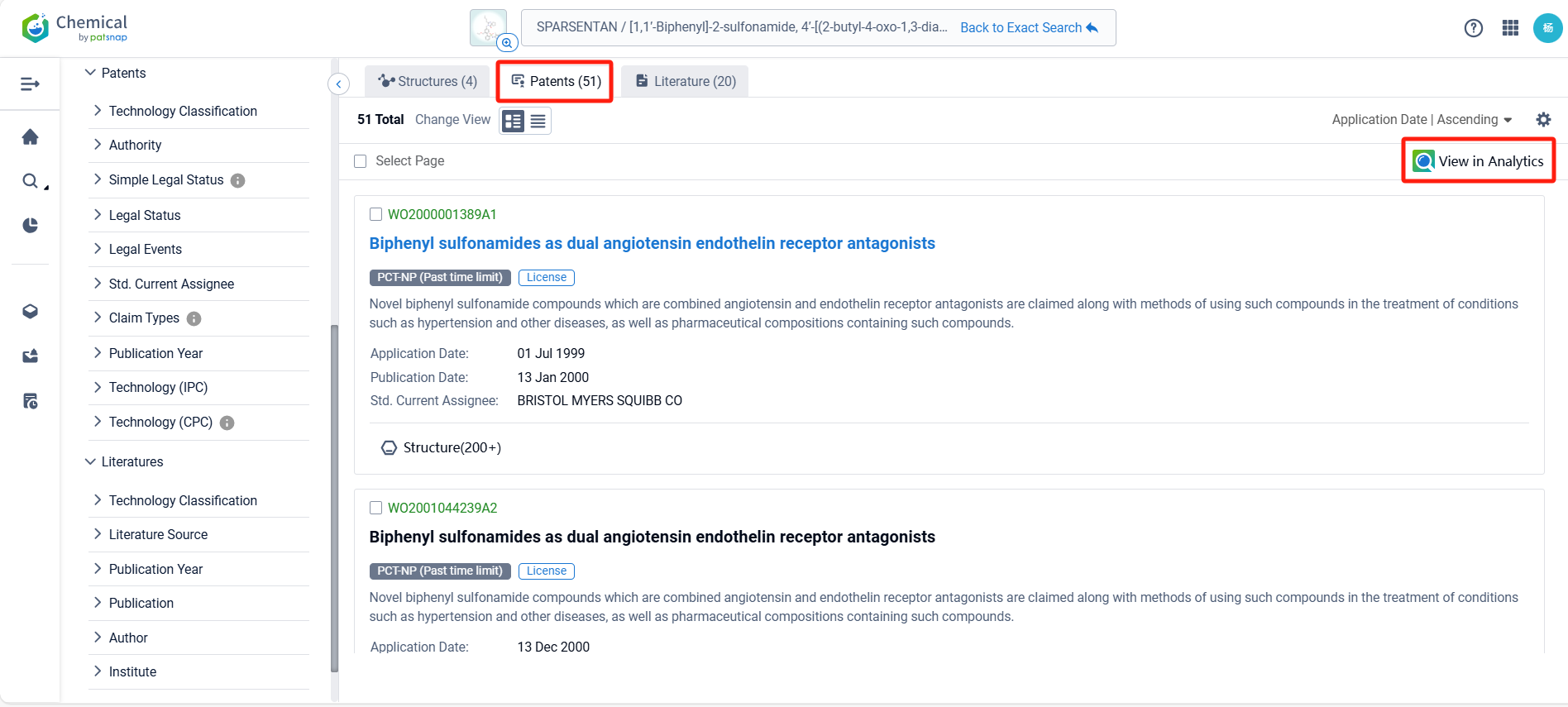
Log in to the Patsnap Chemical. Select the structural search and enter the common identity information of Sparsentan (such as CAS number, generic substance name, molecular formula, SMILES file, etc.). Here, we select "Exact Search", click on search structures, and you can find 51 patents. Clicking the "view in Analytics" will direct you to the Patsnap Patent.
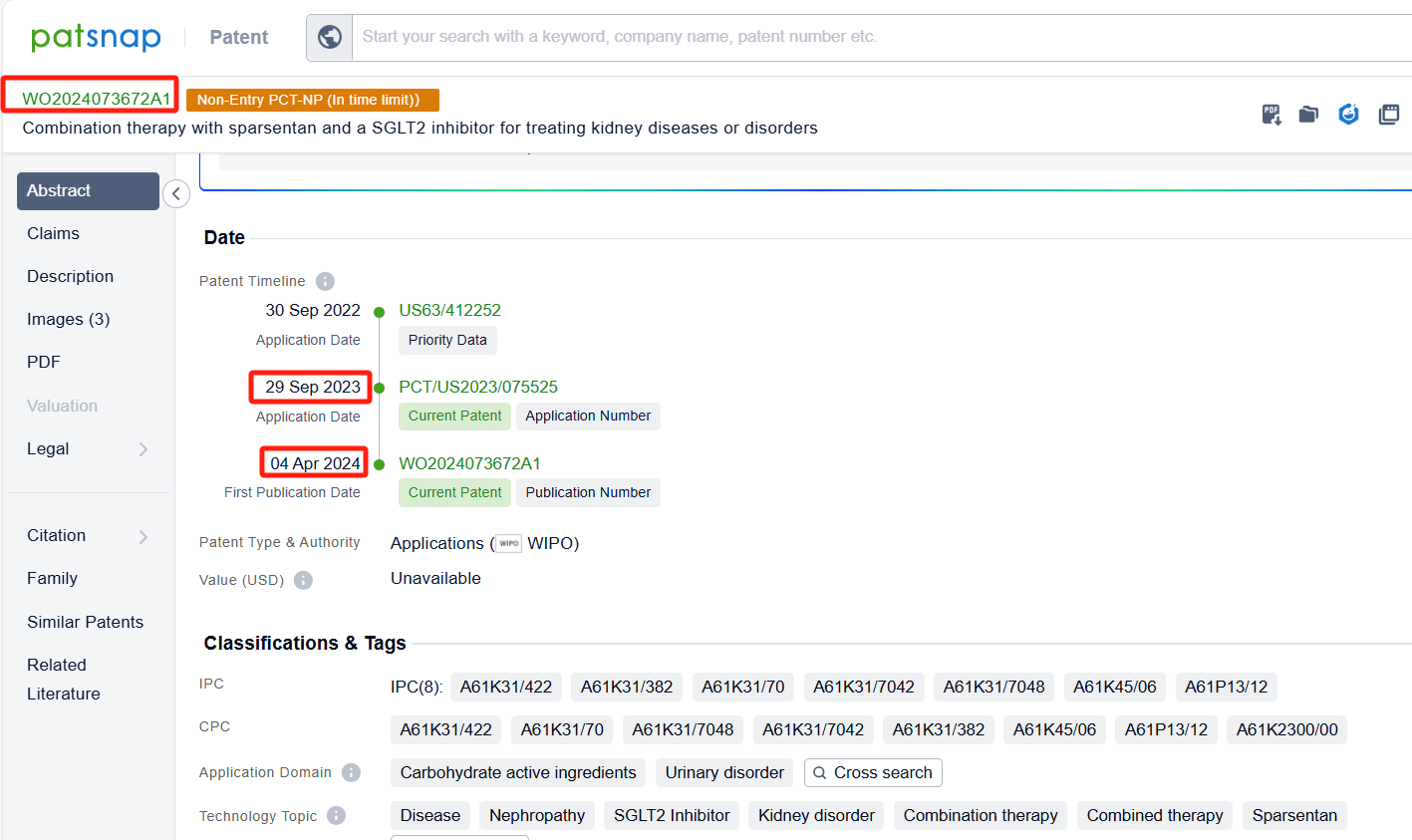
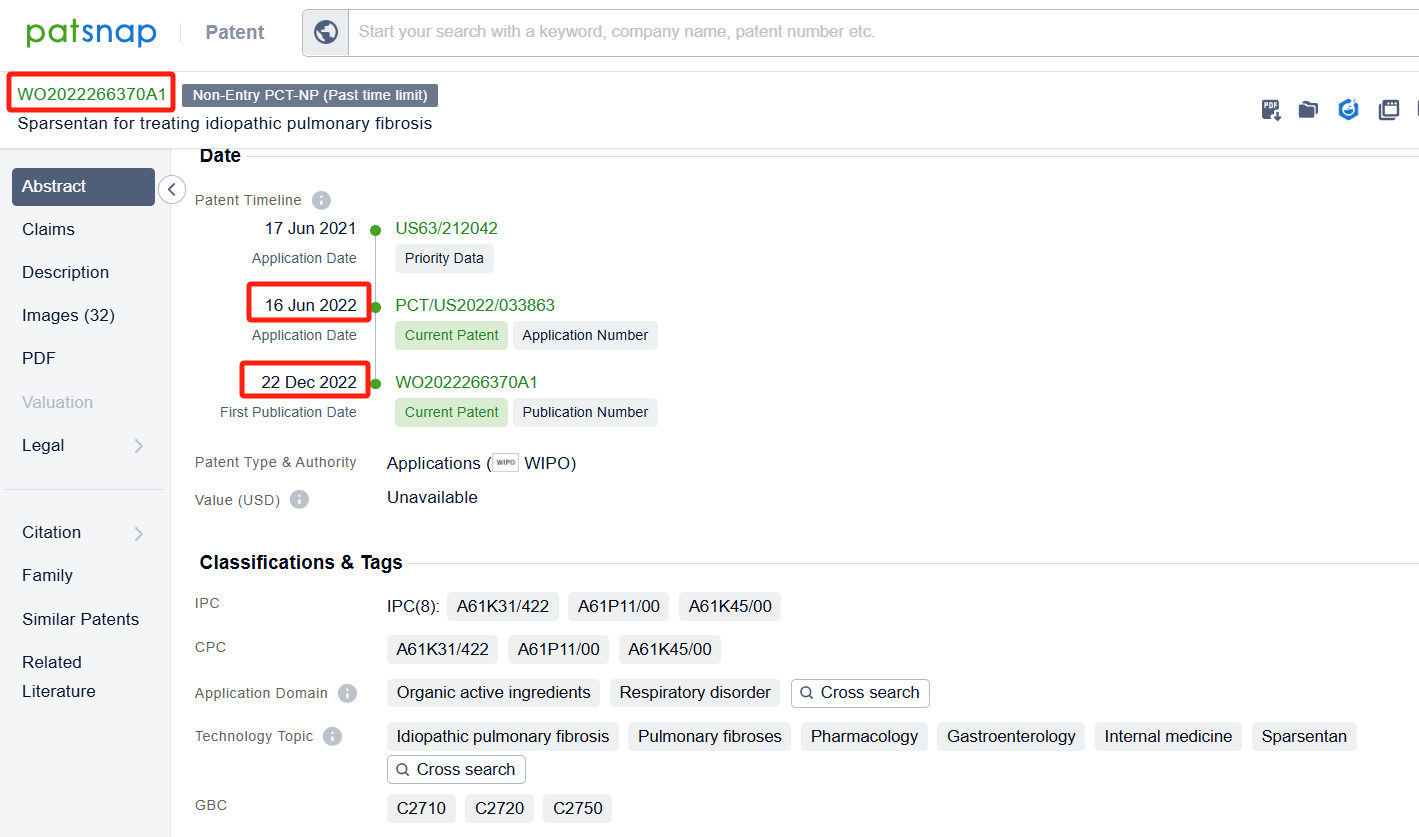
In the Patsnap patent database, we can sort patents by their publication dates to identify the latest patents on Sparsentan. By reviewing the aforementioned patents, we can observe that TRAVERE THERAPEUTICS, INC.'s international patent WO2024073672A1(application date 20230929, publication date 20240404) discusses the use of sparsentan and a sodium-glucose cotransporter-2 (SGLT2) inhibitor for the treatment of kidney diseases or disorders, such as focal segmental glomerulosclerosis (FSGS) and immunoglobulin A nephropathy (IgAN). Additionally, Aria Pharmaceuticals, Inc.'s patent WO2022266370A1(application date 20220616, publication date 20221222) describes methods for treating idiopathic pulmonary fibrosis (IPF) using a dual-acting angiotensin and endothelin receptor antagonist(Sparsentan). The technical effects of the patent include reducing lung inflammation and fibrosis, inhibiting lung myofibroblast phenotypic transition, and treating patients with genetic mutations or smoking.
This information presents Sparsentan as an innovative small molecule drug that has successfully achieved approval for a wide range of therapeutic areas and indications. Its targeting of AT1R x ETA makes it a promising option for the treatment of conditions such as glomerulonephritis and nephrosis. The involvement of a major pharmaceutical company like Bristol Myers Squibb Co. and the regulatory designations of priority review, accelerated approval, and orphan drug status further underscore the significance of this drug in the pharmaceutical industry.
In summary, Sparsentan is a small molecule drug approved for various therapeutic areas and indications, with its originator organization being Bristol Myers Squibb Co. Its approval in the United States in 2023 and the regulatory designations highlight its potential as a valuable addition to the pharmaceutical landscape.
AI built to maximize IP and R&D efficiency
Redefine chemical FTO with a range of structure retrieval options at your fingertips, from exact matches to similarity searches, all powered by deep data processing techniques and proprietary AI algorithms to eliminate the risk of omitting key results.