SpliSense has successfully concluded the Phase 1 clinical trial of SPL84, an RNA-based therapy aimed at treating Cystic Fibrosis
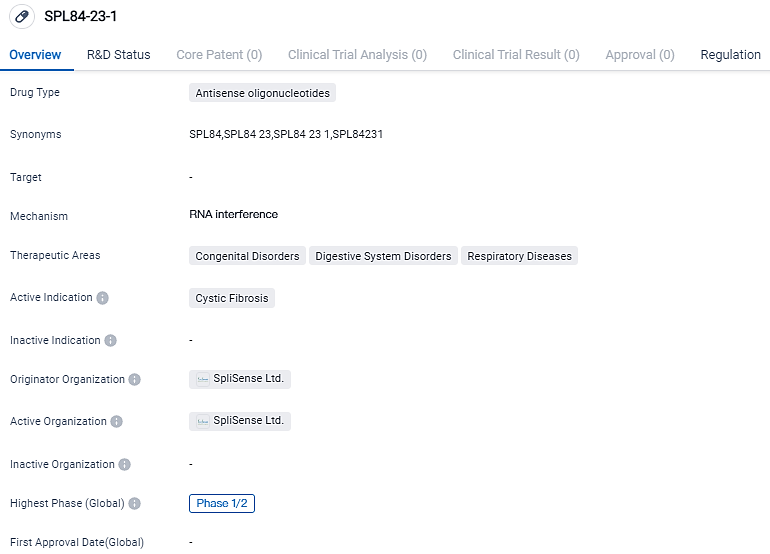
SpliSense, a biopharmaceutical firm focused on the development of innovative RNA-based therapies for pulmonary conditions such as cystic fibrosis, muco-obstructive diseases, and idiopathic pulmonary fibrosis, declared the successful completion of a Phase 1 clinical trial for SPL84, the company's primary inhaled antisense oligonucleotide product designated for the management of CF patients exhibiting the 3849+10 kilobase C->T splicing mutation in the transmembrane conductance regulator gene.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
During the Phase 1 trial, 32 healthy male volunteers were enrolled to receive a single dose of either SPL84 or a placebo. Four different doses of SPL84 were tested: 20, 40, 80, and 160 mg. The results indicated that SPL84 demonstrated a favorable safety profile and was well tolerated, with no serious adverse events or significant adverse events related to the treatment.
There were no significant changes observed in vital signs, clinical laboratory values, ECG, physical examination, or pulmonary function compared to the baseline. The systemic exposure to SPL84 was minimal and dependent on the dose administered.
The Phase 1 study, which was placebo-controlled, double-blind, and randomized, aimed to evaluate the safety, tolerability, and pharmacokinetics of SPL84 in healthy volunteers. The administration of SPL84 was through inhalation as a single ascending dose.
"We are delighted with the positive safety outcomes observed during this Phase 1 study of SPL84, positioning us well for our Phase 2 efficacy study scheduled to commence in the first half of next year," commented Dr. Gili Hart, CEO of SpliSense. "Cystic fibrosis is a prevalent genetic disease that leads to frequent lung infections, breathing difficulties, and reduced life expectancy."
Dr. Hart further added, "The encouraging results from the Phase 1 clinical trial, along with previously published preclinical data in Nucleic Acid Therapeutics demonstrating widespread distribution of SPL84 in the lung, provide strong support for the continued development of our broader pulmonary pipeline.
This includes potential treatments for muco-obstructive diseases and idiopathic pulmonary fibrosis, both of which are expected to enter clinical trials next year."
SpliSense utilizes short, precisely targeted RNA segments called ASOs (antisense oligonucleotides) to address various mutations in CFTR mRNA. Specifically, the ASOs bind specifically to the mutated CFTR RNA in the targeted sequence, leading to regulation of the mutated region within the mRNA and enabling the production of fully functional CFTR proteins by the cell.
The ASOs are administered directly to the lungs via inhalation, with a duration of less than 10 minutes and a dosing frequency of either weekly or every other week. The ASOs have demonstrated efficient uptake by lung cells, promoting the production of corrected CFTR mRNA and ultimately functional CFTR proteins.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
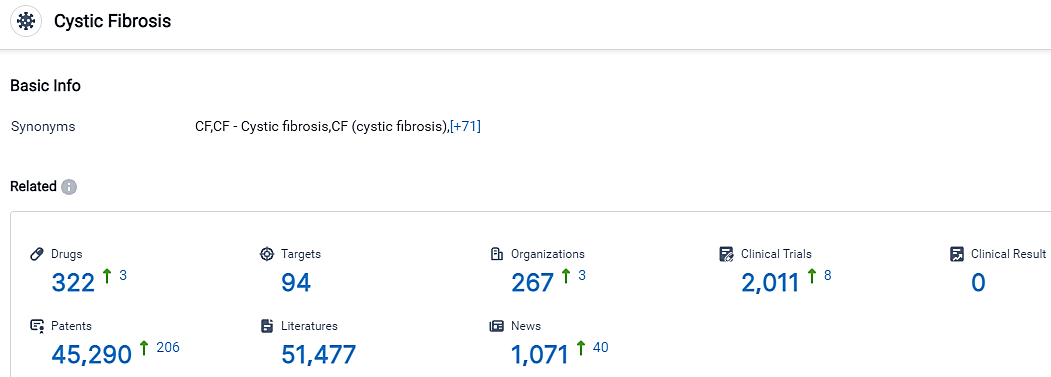
According to the data provided by the Synapse Database, As of September 11, 2023, there are 322 investigational drugs for Cystic Fibrosis, including 94 targets, 267 R&D institutions involved, with related clinical trials reaching 2011, and as many as 45290 patents.
ertex Pharmaceuticals, Inc. is the top player in the field of drug development for Cystic Fibrosis. CFTR remains the primary protein targeted, and small molecule drugs stand as the prevailing drug category. There exists a highly competitive environment in the Cystic Fibrosis sector, with numerous companies and drug classifications contending for market dominance. The future advancement of this indication holds great potential, as ongoing research and development endeavors strive to enhance treatment alternatives for individuals afflicted with Cystic Fibrosis.