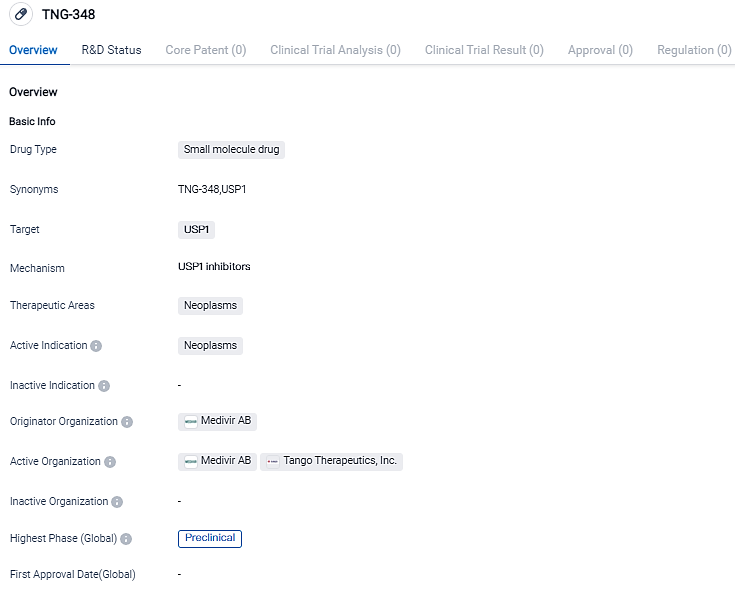
Tango Therapeutics receives FDA approval for TNG348, a new USP1 inhibitor targeting BRCA1/2-mutant and other HRD+ cancers
Tango Therapeutics, a biotech company focused on precision cancer medicines, has received FDA clearance for its IND application for TNG348, a new USP1 inhibitor designed to treat BRCA1/2 mutant and other HRD+ cancers.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"The FDA's approval to begin the TNG348 phase 1/2 clinical study marks a significant milestone in the development of an innovative treatment that has the potential to effectively address a considerable number of ovarian, prostate, and breast cancers. Our plan is to initiate the TNG348 clinical trial in the first half of 2024," stated Barbara Weber, MD, President and CEO of Tango Therapeutics.
Previous studies have shown that USP1 inhibition impedes DNA repair through a mechanism distinct from PARP inhibitors. TNG348 has shown activity in xenografts that have both primary and acquired resistance to PARP inhibitors.
Additionally, preclinical data demonstrates that USP1 has a synergistic effect with PARP inhibitors in xenograft models that are naïve to PARP inhibitor therapy. These findings suggest that TNG348 may provide benefits to patients who have progressed on a PARP inhibitor or can be used in combination with single-agent PARP inhibitor therapy," added Barbara Weber.
The phase 1/2 clinical trial will evaluate the safety, pharmacokinetics, pharmacodynamics, and efficacy of TNG348 as a standalone treatment and in combination with olaparib, a PARP inhibitor, in patients with BRCA1/2-mutant and other HRD+ cancers. HRD+ cancers, which include BRCA1/2 mutations, are responsible for approximately 50% of ovarian cancers, 25% of breast cancers, 10% of prostate cancers, and 5% of pancreatic cancers..
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
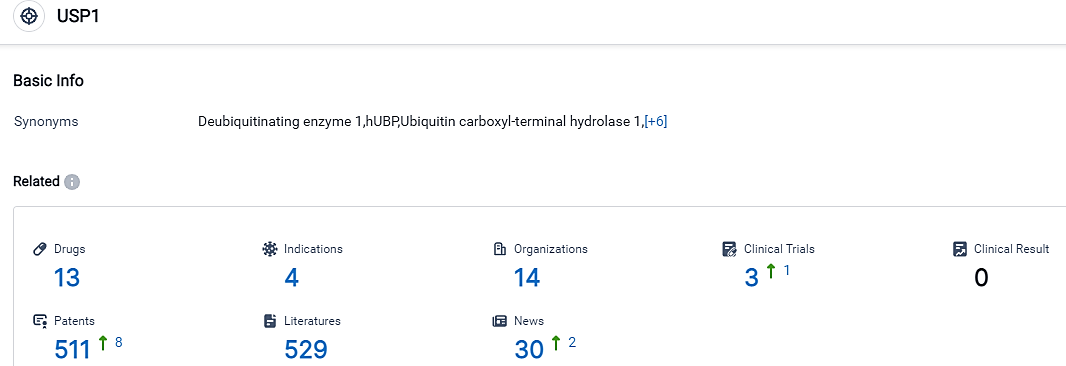
According to the data provided by the Synapse Database, As of September 11, 2023, there are 13 investigational drugs for the USP1 target, including 4 applicable indications,14 R&D institutions involved, with related clinical trials reaching 3,and as many as 511 patents.
The evaluation of the USP1 target showcases a competitive environment with several companies actively engaged in research and development activities. InSilico Medicine Hong Kong Ltd. stands out by having the most extensive portfolio of drugs in Phase 1 and Preclinical stages, signifying substantial progress in R&D. The indications targeted by these drugs include Solid Tumors, Advanced Malignant Solid Neoplasm, Neoplasms, and HRD positive cancer, with the highest number of drugs found in the Phase 1 and Preclinical stages. On the whole, the USP1 target displays promising potential for development and ongoing research in the pharmaceutical industry.