TAR-200 Monotherapy Yields High Remission Rates in High-Risk Non-Muscle-Invasive Bladder Cancer
Johnson & Johnson released new findings from the second cohort of the Phase 2b SunRISe-1 trial, which focuses on the effectiveness and safety of the experimental TAR-200 monotherapy. This study involves patients diagnosed with high-risk non–muscle-invasive bladder cancer with carcinoma in situ who are either ineligible for or have opted not to undergo radical cystectomy. These findings were highlighted in a plenary session at the 2024 American Urological Association Annual Meeting, held from May 3-6 in San Antonio, Texas.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Joseph Jacob, M.D., M.S., from the Department of Urology at Upstate Medical University and the lead presenter, commented, "The observed high rates of complete response and their sustainability in patients receiving TAR-200 highlight its promising potential in treating BCG-unresponsive HR-NMIBC." He noted, "Such outcomes fulfill a substantial need for conservative bladder treatments within these patient groups."
The investigation included 85 patients administered TAR-200 as a sole treatment. Central evaluation proved the complete response rate to be 82.8%, determined through urine cytology or biopsy. According to the study guidelines, which align with U.S. Food and Drug Administration regulations, there was no second line of treatment for those who did not initially respond. It was noted that the estimated one-year response period was 74.6%. At the median observation point of 29.9 weeks for those responding, 41 out of 48 were still in complete response by January 2, 2024. Additionally, no cases had advanced to muscle-invasive bladder cancer or showed signs of spreading.
At the initial assessment in week 12, 98% of the complete responses were recorded, and among five patients who reached two years of treatment, four maintained their complete response status. The rates assessed by investigators were closely aligned with the central observations.
Christopher Cutie, M.D., Vice President and Disease Area Leader of Bladder Cancer at Johnson & Johnson Innovative Medicine, stated, "These findings are crucial in our pursuit to introduce new therapeutic options focusing on bladder conservation and prolonged survival." He emphasized, "The data underscore TAR-200’s capability to revolutionize treatment approaches and our continual commitment to meet the needs of patients contending with this difficult condition."
TAR-200 is an experimental system developed to sustain the release of gemcitabine locally within the bladder. The installation occurs in an outpatient office setting during a brief procedure lasting 3 to 5 minutes, without the need for anesthesia. In December 2023, the FDA awarded TAR-200 a Breakthrough Therapy Designation for its potential as a future option for patients with BCG-unresponsive HR-NMIBC who cannot undergo or choose not to have radical cystectomy.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
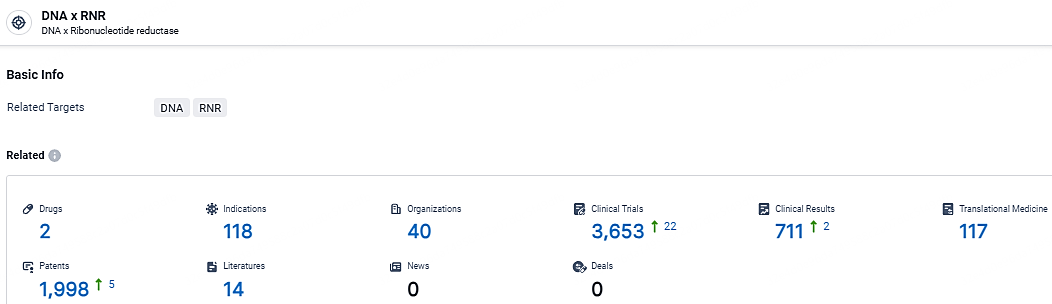
According to the data provided by the Synapse Database, As of May 6, 2024, there are 2 investigational drugs for the DNA and RNR target, including 118 indications, 40 R&D institutions involved, with related clinical trials reaching 3653, and as many as 1998 patents.
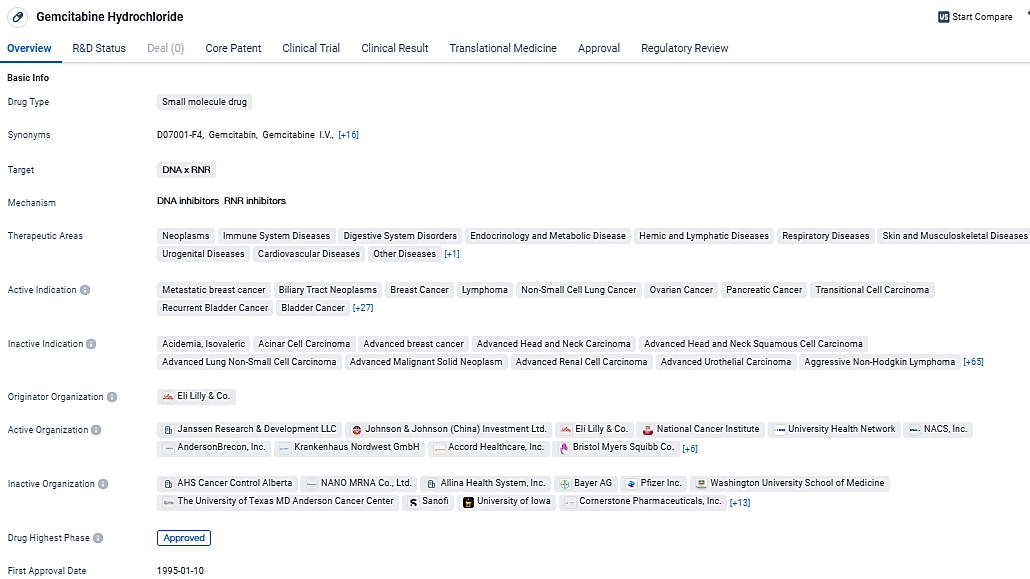
Gemcitabine Hydrochloride is a versatile drug that targets DNA and RNR, making it effective in treating various types of cancer and other diseases. Its approval in multiple countries and designations as a breakthrough therapy and orphan drug highlight its potential in the pharmaceutical industry.