Targeted immune modulator: TNF inhibitor
Tumor Necrosis Factor (TNF) is a protein produced by immune cells in the human body. It plays a crucial role in regulating inflammation and immune responses.
TNF acts as a signaling molecule, binding to specific receptors on cells and triggering a cascade of events that promote inflammation. It helps in the defense against infections and plays a role in the destruction of cancer cells.
However, excessive or prolonged TNF activity can lead to chronic inflammation and contribute to various diseases, including autoimmune disorders like rheumatoid arthritis and inflammatory bowel disease. Pharmaceutical interventions targeting TNF have been developed to modulate its activity and treat these conditions.
TNF Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 3 Sep 2023, there are a total of 83 TNF drugs worldwide, from 100 organizations, covering 90 indications, and conducting 843 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.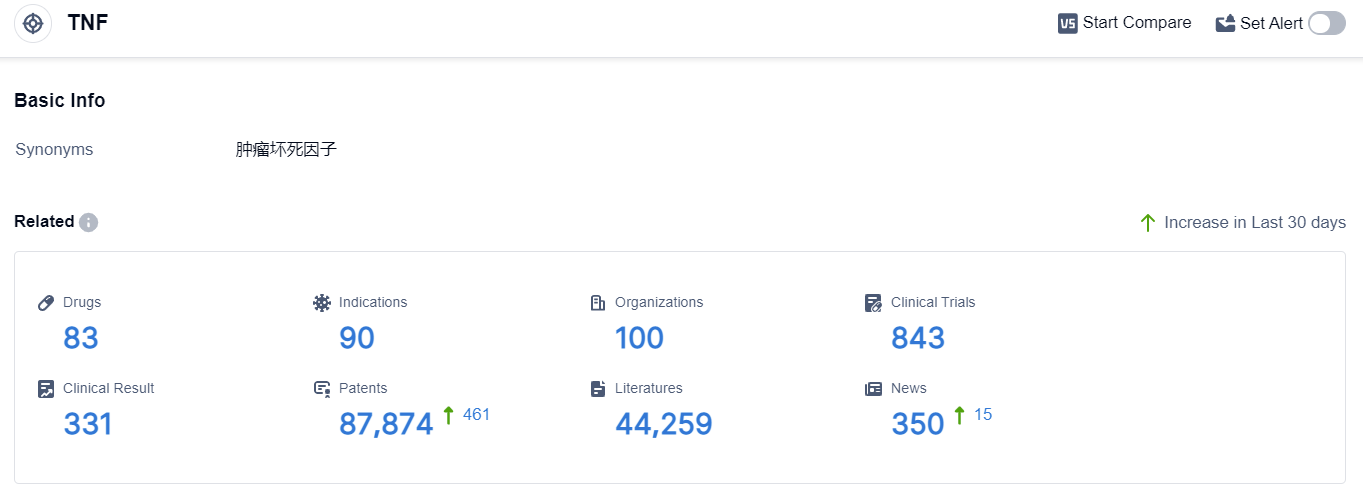
The analysis of the target TNF from various perspectives provides valuable insights into the current competitive landscape and future development. Bristol Myers Squibb Co., INmune Bio, Inc., and other companies are actively involved in the development of TNF drugs. Multiple indications have approved drugs, indicating the potential therapeutic benefits of targeting TNF. Small molecule drugs, molecular glues, and biosimilars are progressing rapidly, suggesting intense competition in the market. Japan, the United States, and the European Union are leading in terms of approved drugs, with China also making progress. This analysis sets the stage for further research and development in the field of TNF-targeted therapies.
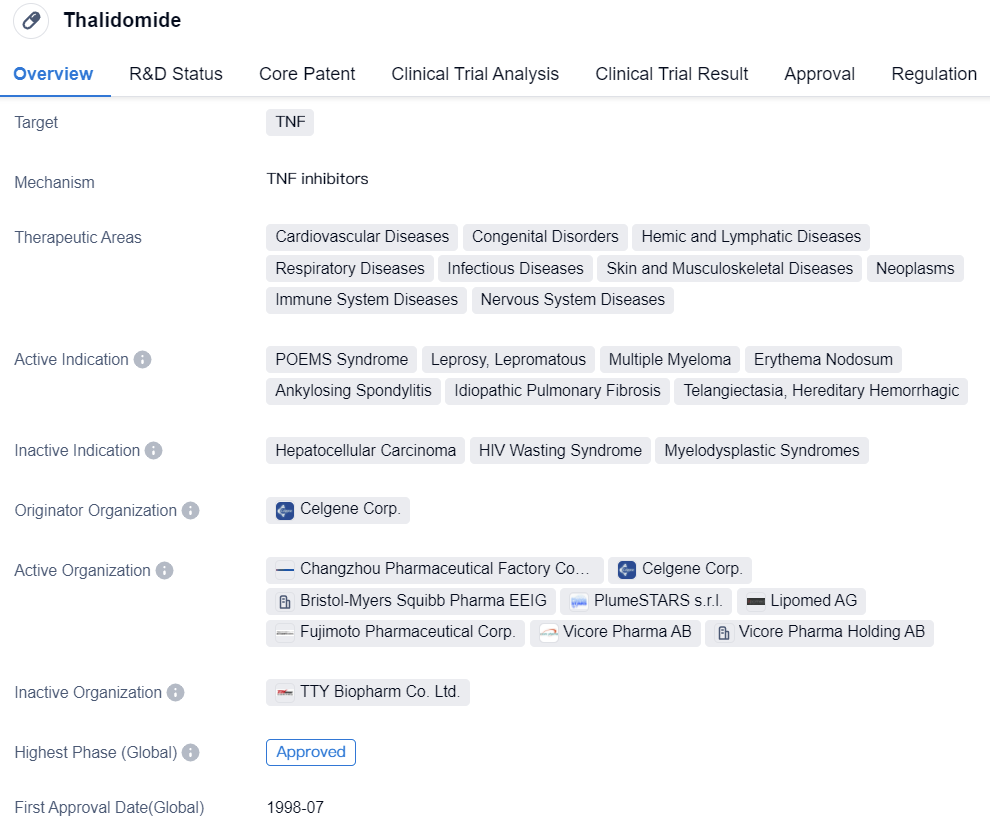
Key Drug: Thalidomide
Thalidomide is a small molecule drug classified as a molecular glue that primarily targets TNF (tumor necrosis factor). It has been approved for use in various therapeutic areas, including cardiovascular diseases, congenital disorders, hemic and lymphatic diseases, respiratory diseases, infectious diseases, skin and musculoskeletal diseases, neoplasms, immune system diseases, and nervous system diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The drug has shown efficacy in treating several active indications, such as POEMS syndrome, leprosy (lepromatous), multiple myeloma, erythema nodosum, ankylosing spondylitis, idiopathic pulmonary fibrosis, and telangiectasia, hereditary hemorrhagic. Thalidomide's approval for these indications has been granted by regulatory authorities in multiple countries, including the United States.
Celgene Corp. is the originator organization of Thalidomide, which has successfully developed and brought the drug to market. Thalidomide has reached the highest phase of development, with global and Chinese approvals. The drug received its first approval in the United States in July 1998.
Thalidomide's regulatory status includes accelerated approval and orphan drug designation. Accelerated approval allows for the expedited review and approval of drugs that address unmet medical needs, while orphan drug designation provides incentives for the development of drugs targeting rare diseases.
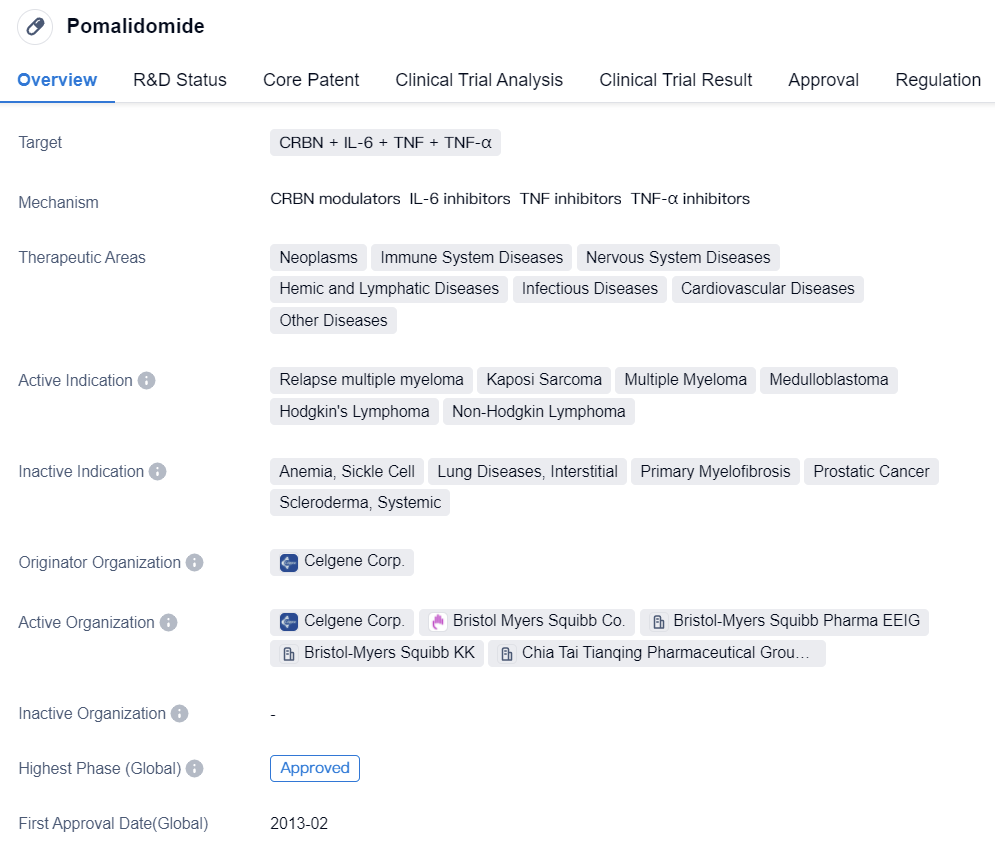
Pomalidomide
Pomalidomide is a small molecule drug classified as a molecular glue. It targets several proteins including CRBN, IL-6, TNF, and TNF-α. The drug has shown potential in treating various therapeutic areas such as neoplasms, immune system diseases, nervous system diseases, hemic and lymphatic diseases, infectious diseases, cardiovascular diseases, and other diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Pomalidomide has been indicated for the treatment of relapse multiple myeloma, Kaposi Sarcoma, multiple myeloma, medulloblastoma, Hodgkin's Lymphoma, and non-Hodgkin lymphoma. It was developed by Celgene Corp., a pharmaceutical company known for its expertise in the field of biomedicine.
The drug has received approval for its highest phase in both the global market and China. Its first approval was granted in the United States in February 2013. Pomalidomide has undergone various regulatory processes, including priority review, accelerated approval, fast track, and orphan drug designation.
Overall, Pomalidomide's approval and its broad therapeutic areas of application make it a promising drug in the pharmaceutical industry. Its development by Celgene Corp., a reputable organization in the field, further adds to its credibility. Further research and clinical trials may provide more insights into its efficacy and potential applications in the future.