Telix Announces Positive rPFS Results from ProstACT SELECT Trial for Prostate Cancer Therapy TLX591
Telix Pharmaceuticals Limited reports further encouraging results from the ProstACT SELECT clinical trial involving TLX591 (177Lu rosopatamab tetraxetan), a lutetium-labeled rADC treatment designed for adult patients with PSMA-positive metastatic castration-resistant prostate cancer.
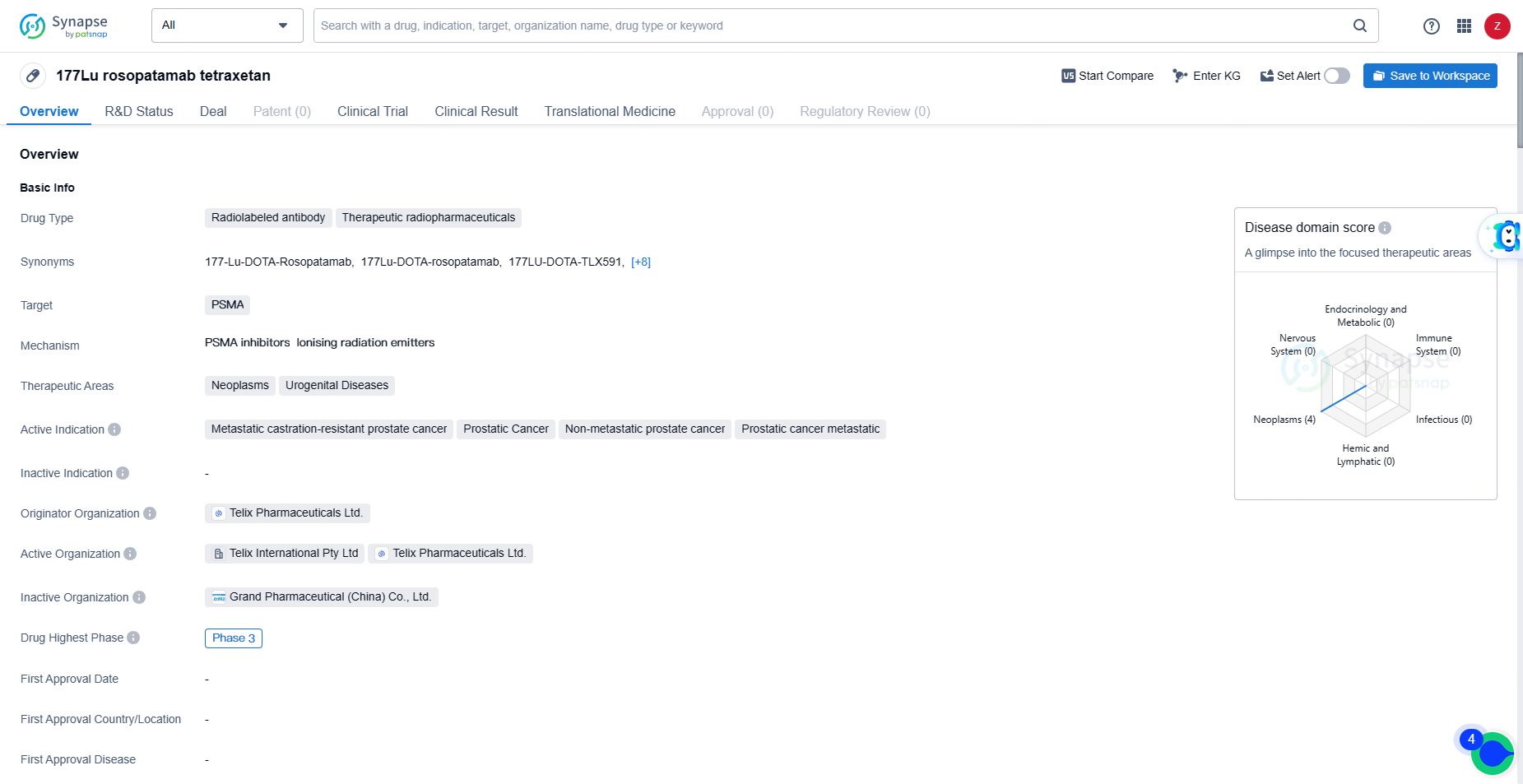
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
SELECT is a radiogenomics investigation aimed at assessing lesion concordance between 68Ga (gallium)-based PSMA-PET imaging and TLX591 dosimetry, in order to validate PET imaging for selecting patients for rADC therapy. The Company has previously reported the final safety data from this study.
The investigation has shown a median rPFS of 8.8 months, indicating a promising signal for the potential effectiveness of TLX591 in this patient group. The evaluable sample size for rPFS included 23 patients with previously treated, progressive mCRPC who received two 76 mCi intravenous infusions of TLX591, spaced 14 days apart.
The SELECT study involved a diverse group of patients with low, medium, and high disease burden to enable imaging cross-comparison, with most having undergone two prior lines of therapy.
Nat Lenzo, MD, a Nuclear Oncologist and General Internal Medicine Physician and lead recruiter for the SELECT trial, remarked, “We are encouraged by this rPFS outcome, which compares favorably to small molecule radioligand therapy Phase I and II studies at similar stages of development. This indicates a compelling signal for the potential efficacy of TLX591 in this heavily pre-treated population.”
Dr. David N. Cade, MD, Group Chief Medical Officer at Telix, stated, “TLX591 is a radio-ADC with substantial potential benefits compared to small molecule radiopharmaceuticals in treating prostate cancer. TLX591 stands out with a patient-friendly dosing schedule that results in significantly lower cumulative radiation exposure compared to small molecule radioligand therapies. This positive efficacy signal from SELECT builds on previous studies that showed TLX591's potential to enhance quality of life and provide durable tumor control in this advanced patient population.”
TLX591 is undergoing further evaluation in the Phase III ProstACT GLOBAL trial for first and second-line mCRPC, which is currently preparing to enroll patients at its initial U.S. sites. This inventive trial design offers physicians a choice between androgen receptor inhibition or docetaxel chemotherapy, integrating with real-world standard of care and reflecting Telix’s ongoing innovation in prostate cancer treatment and commitment to patient outcomes.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
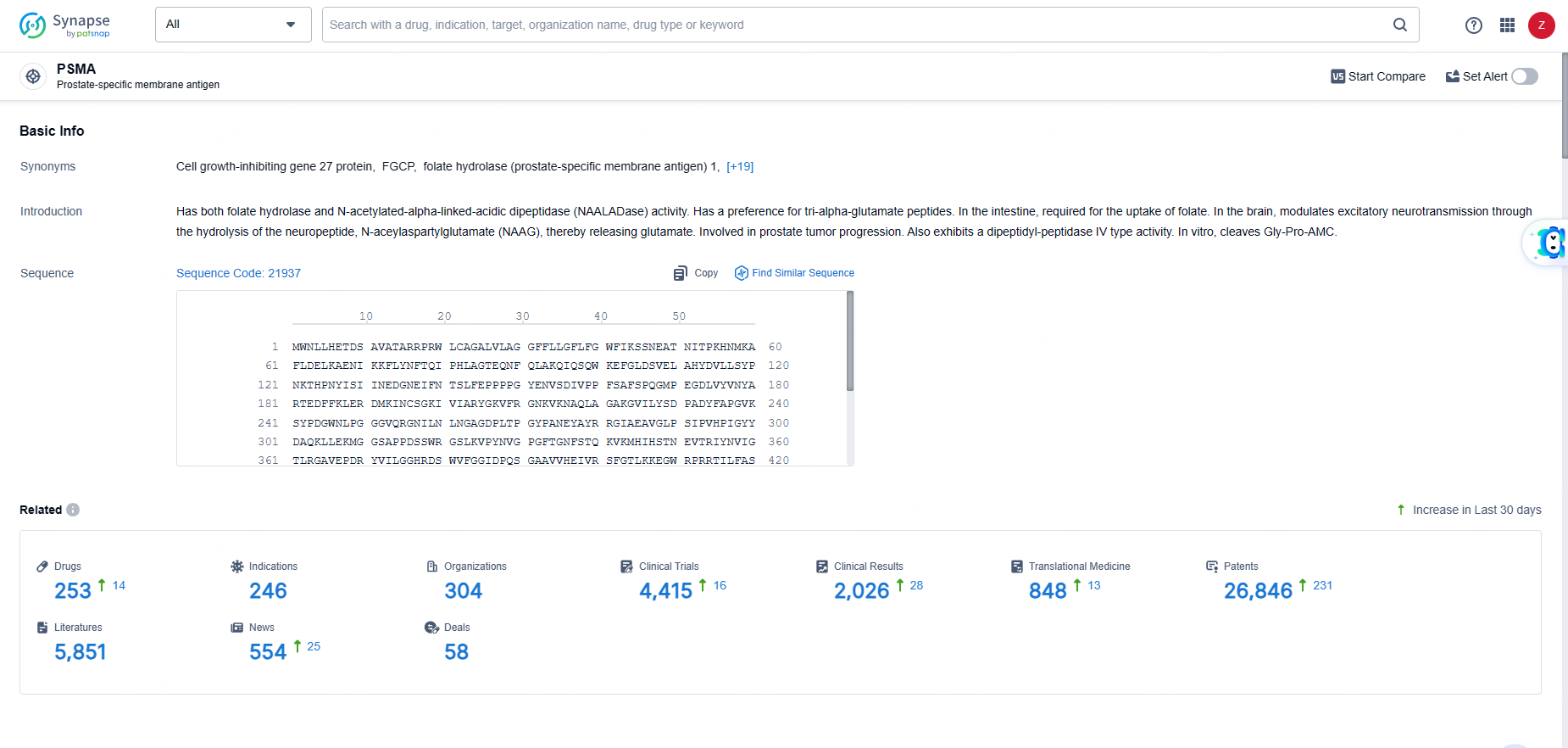 According to the data provided by the Synapse Database, As of June 5, 2024, there are 253 investigational drugs for the PSMA targets, including 246 indications, 304 R&D institutions involved, with related clinical trials reaching 4415, and as many as 26846 patents.
According to the data provided by the Synapse Database, As of June 5, 2024, there are 253 investigational drugs for the PSMA targets, including 246 indications, 304 R&D institutions involved, with related clinical trials reaching 4415, and as many as 26846 patents.
TLX591 represents a significant advancement in the field of biomedicine, particularly in the area of therapeutic radiopharmaceuticals for prostate cancer. As the drug continues to progress through clinical development, it has the potential to make a meaningful impact on the treatment landscape for patients with prostate cancer, offering new hope for improved outcomes and quality of life.