Tepotinib Hydrochloride Hydrate: brief review of its R&D progress and the clinical result in 2023 ESMO
On 23 Oct 2023, liquid biopsies (LBx) and tissue biopsies (TBx) for identifying MET exon 14 (METex14) skipping in advanced NSCLC: Analyses from the phase II VISION study of tepotinib was reported at the ESMO Congress.
Tepotinib Hydrochloride Hydrate's R&D Progress
Tepotinib Hydrochloride Hydrate is a small molecule drug that targets c-Met, a protein involved in cell growth and survival. It has shown potential therapeutic benefits in the treatment of various neoplasms, digestive system disorders, and respiratory diseases. The active indications for this drug include non-small cell lung cancer, colorectal cancer, liver cancer, and hepatocellular carcinoma.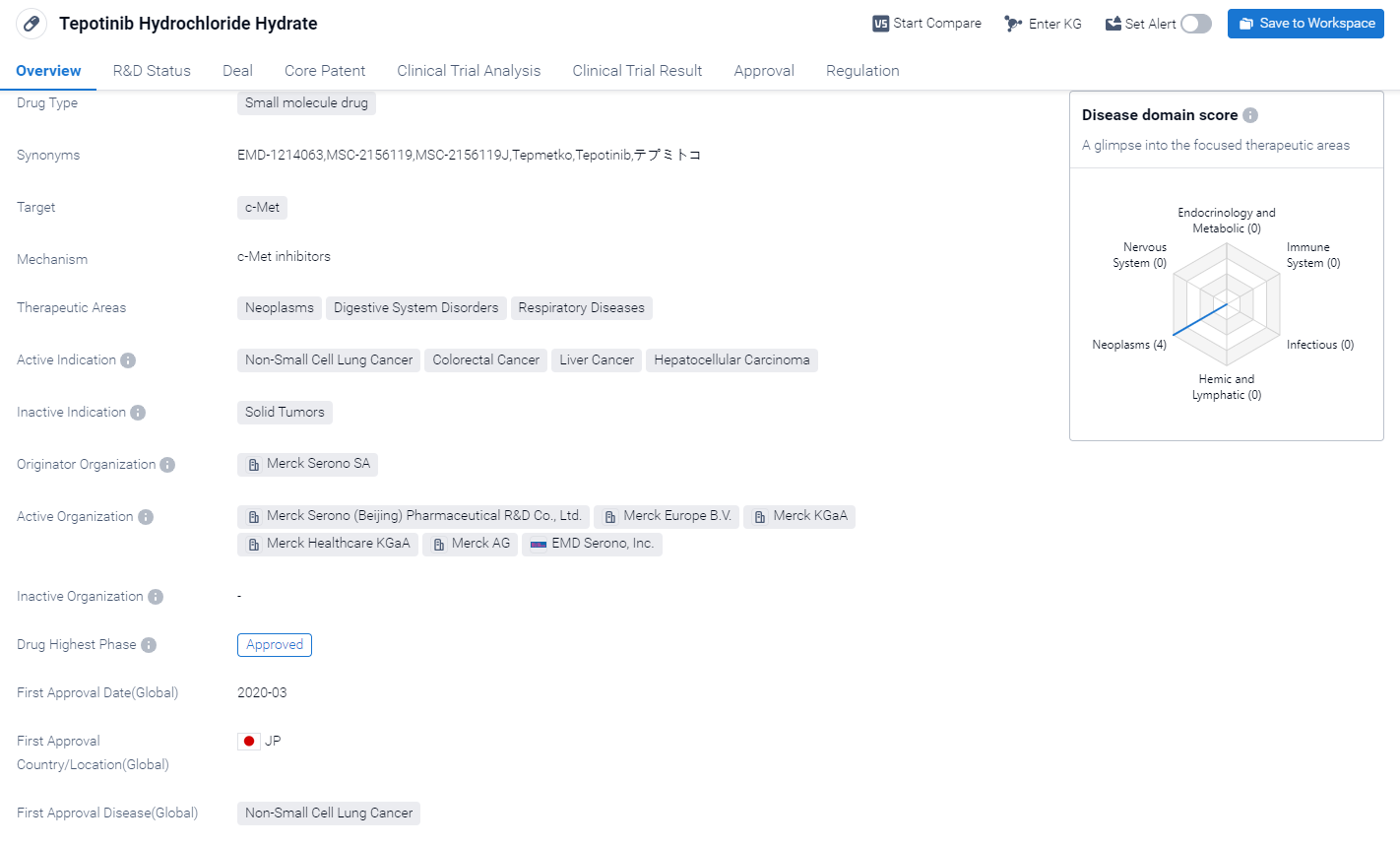
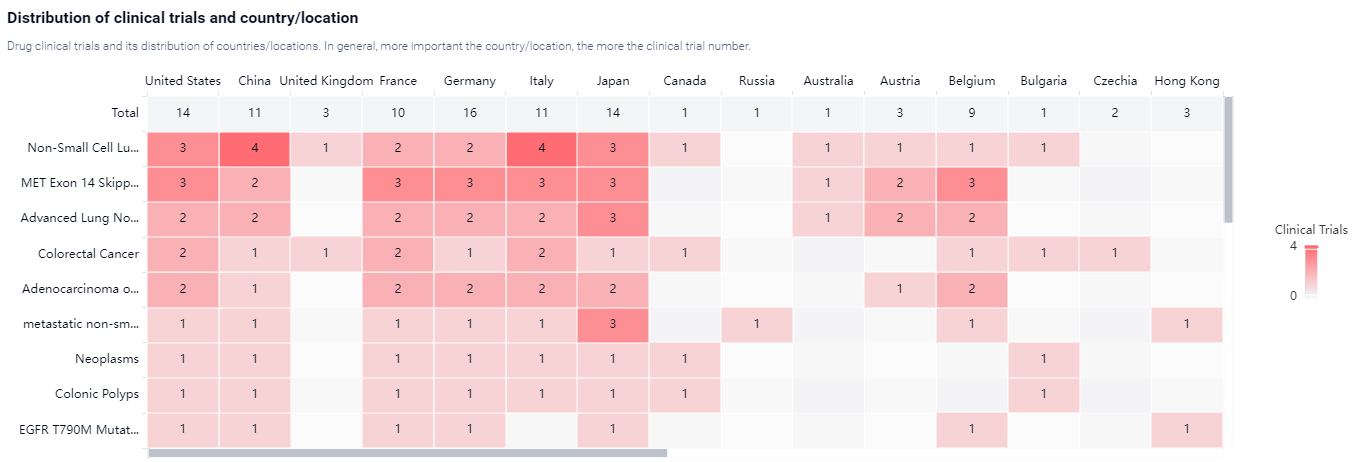
According to the Patsnap Synapse, Tepotinib Hydrochloride HydrateIt has reached the highest phase of development, which is approved globally. And the clinical trial areas for Tepotinib Hydrochloride Hydrate are primarily in the United States, China, and Italy. The key indication is Non-Small Cell Lung Cancer.
Detailed Clinical Result of Tepotinib Hydrochloride Hydrate
This single group assignment, open-labeled clinical trial (NCT02864992) was conducted in advanced NSCLC.
In this study, METex14 was centrally assessed in prescreening by NGS analysis of fresh LBx (Guardant360® or Archer®MET) and/or archival or fresh TBx (Oncomine Focus or Archer®MET). Pts in Japan could enroll based on local TBx RT-PCR via the LC-SCRUM program. Parallel LBx/TBx testing was recommended but not mandatory. Eligibility required LBx-positive (L+) and/or TBx-positive (T+) status.
The result showed that of 313 pts, 208 (66.5%) were T+ and 178 (56.9%) were L+. L+ pts had worse baseline prognostic features than T+ pts, including more pts with ≥3 target lesions (27.5% vs 18.8%) or ECOG PS 1 (76.4% vs 72.1%), higher median sum of target lesion diameters (67.1 vs 55.2 mm) and worse health-related quality of life (mean EORTC QLQ-C30 GHS, 53.9 vs 60.1). In 180 T+ pts with matching LBx results, METex14 was detected in ctDNA (L+) in 74 (41.1%) (i.e. T+/L+) and was undetectable (L–) in 106 (58.9%) (i.e. T+/L–). ORRs were slightly higher in T+/L+ pts, but T+/L– pts had longer DOR, PFS and OS..
It can be concluded that Tepotinib had robust and durable activity in T+ pts with L– or L+ status. While both LBx and TBx are suitable and complementary for detecting METex14, LBx may preferentially select pts with a poorer prognosis and higher tumor load. Undetectable METex14 in baseline ctDNA may define a more favorable treatment outcome. Differences in populations identified by TBx and LBx should be considered when interpreting trial data.
How to Easily View the Clinical Results Using Synapse Database?
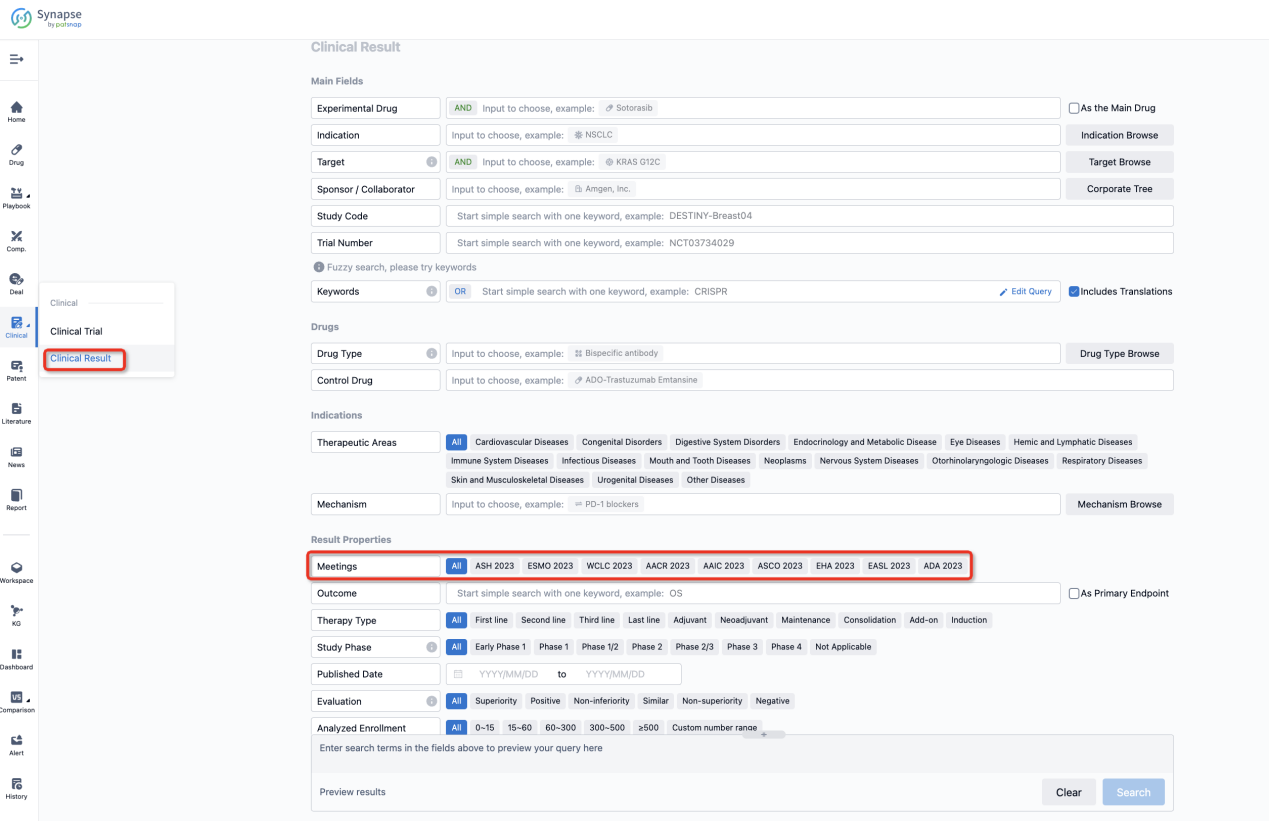
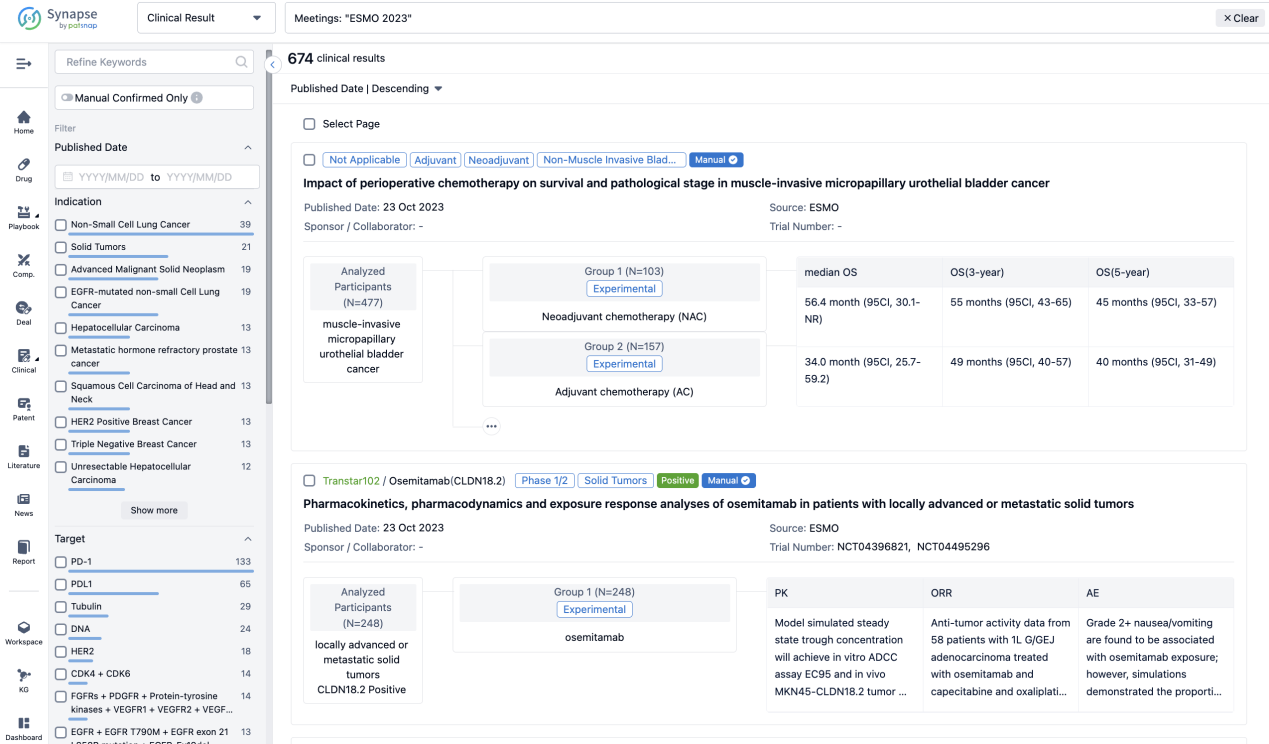
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
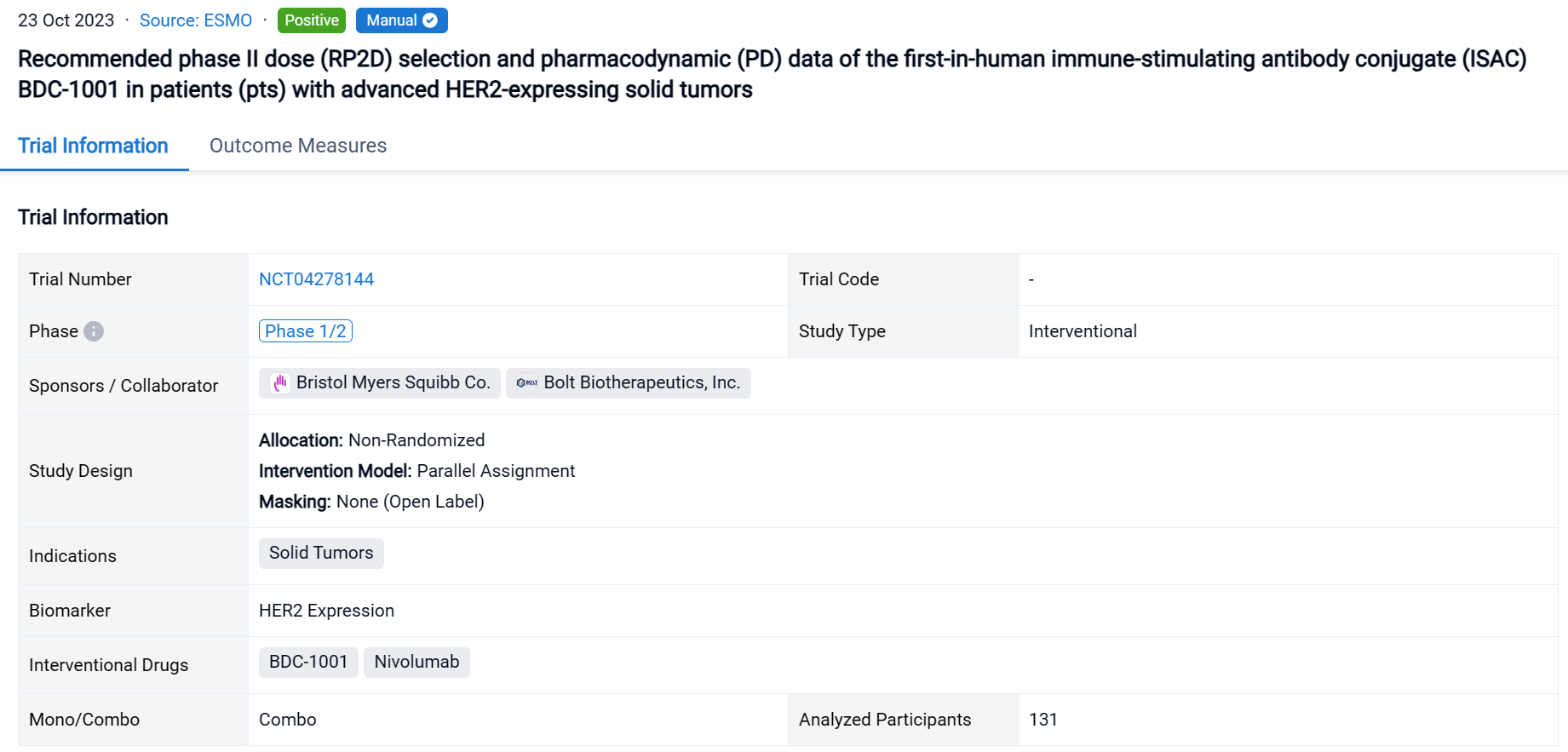
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
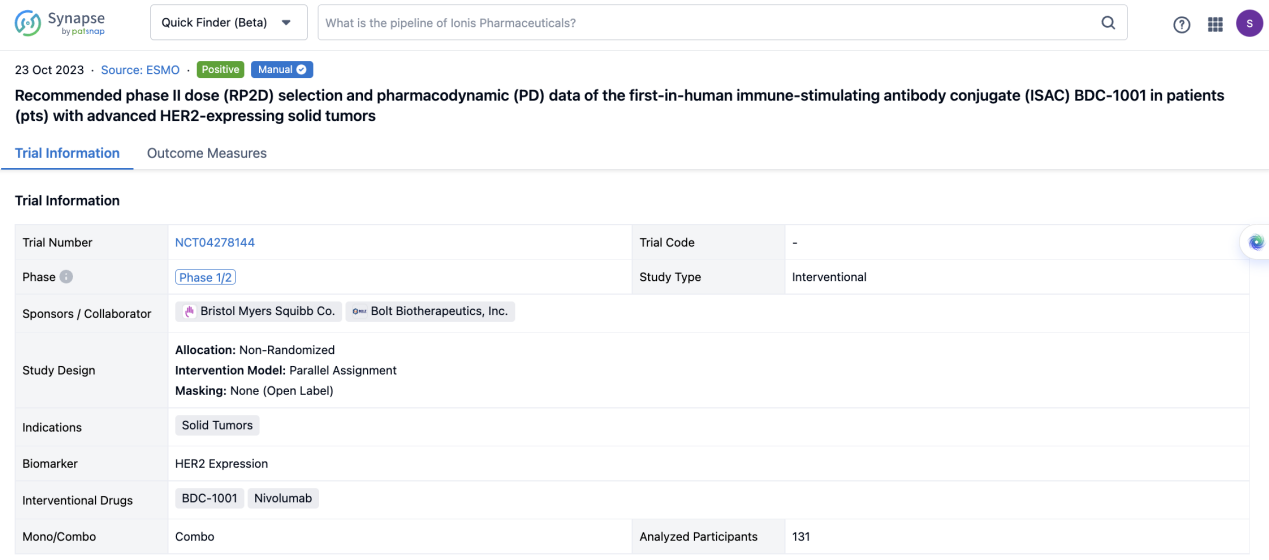
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
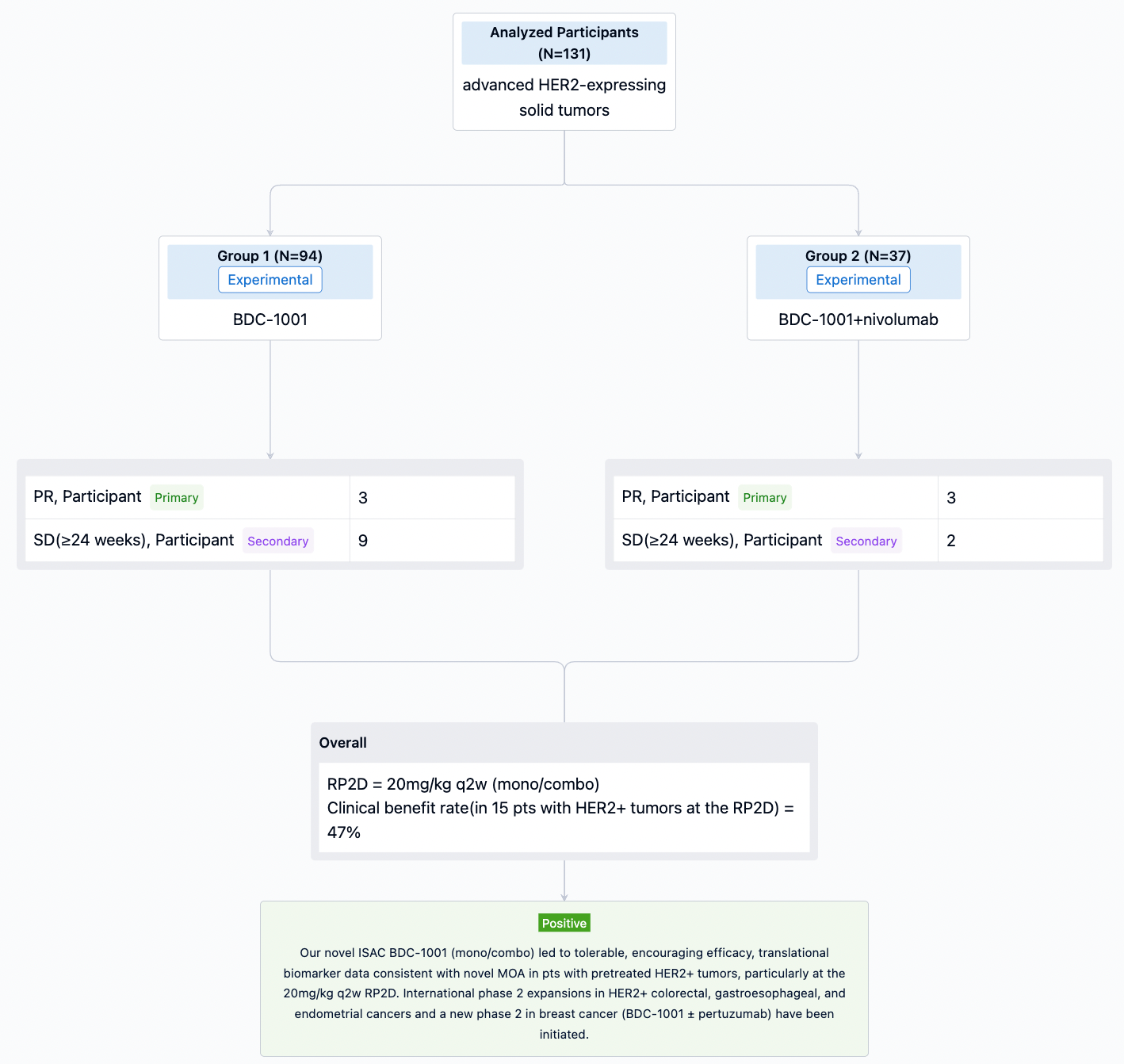
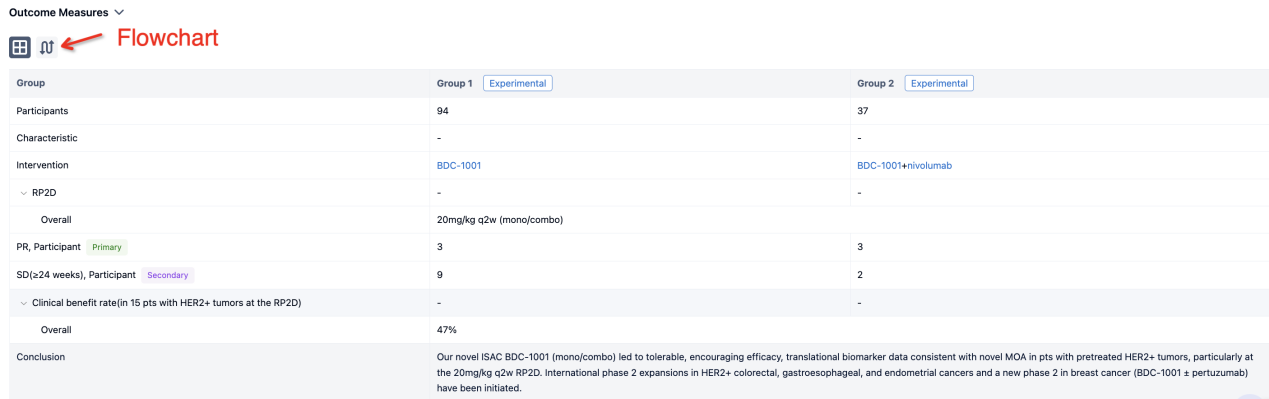
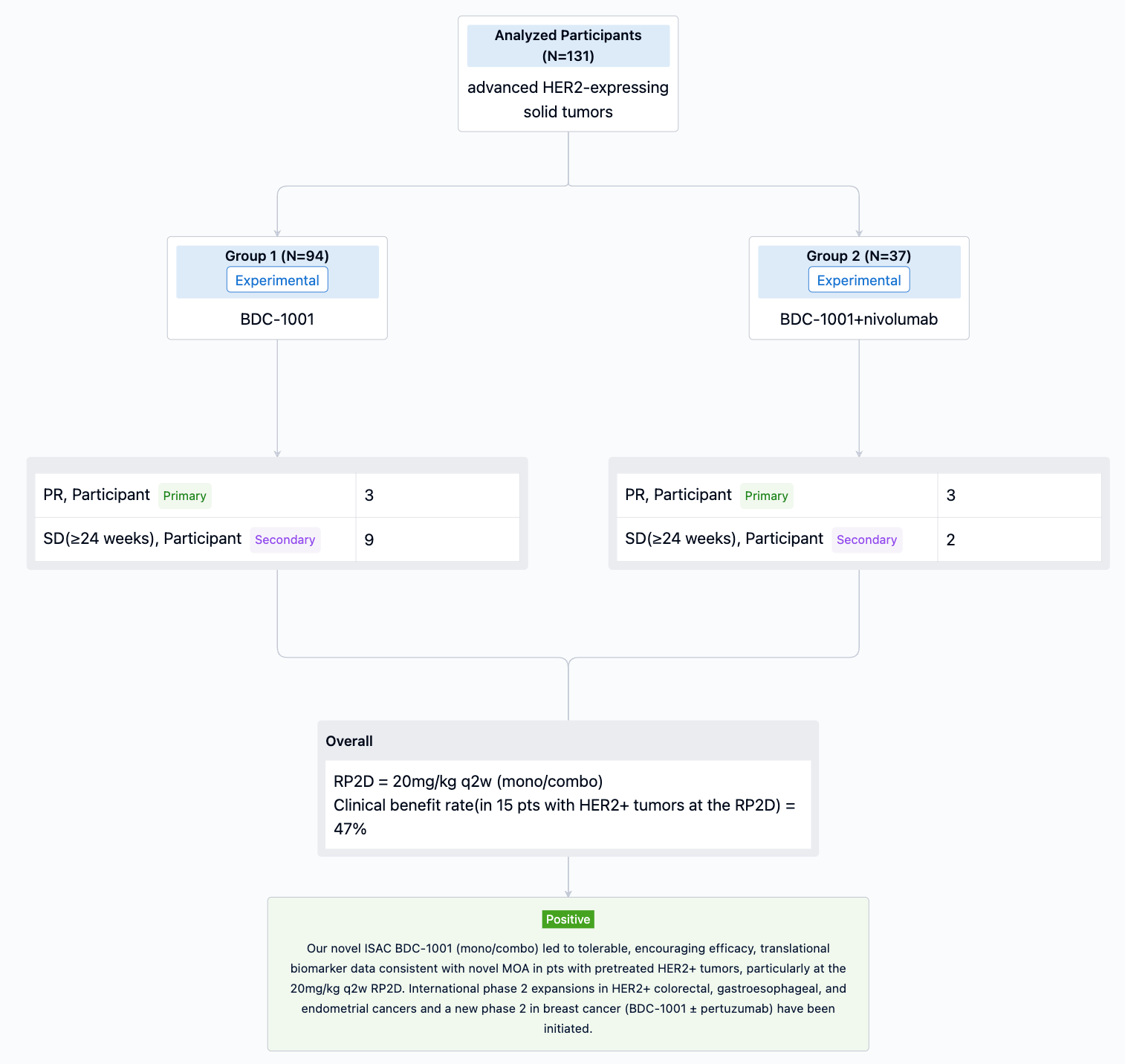
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
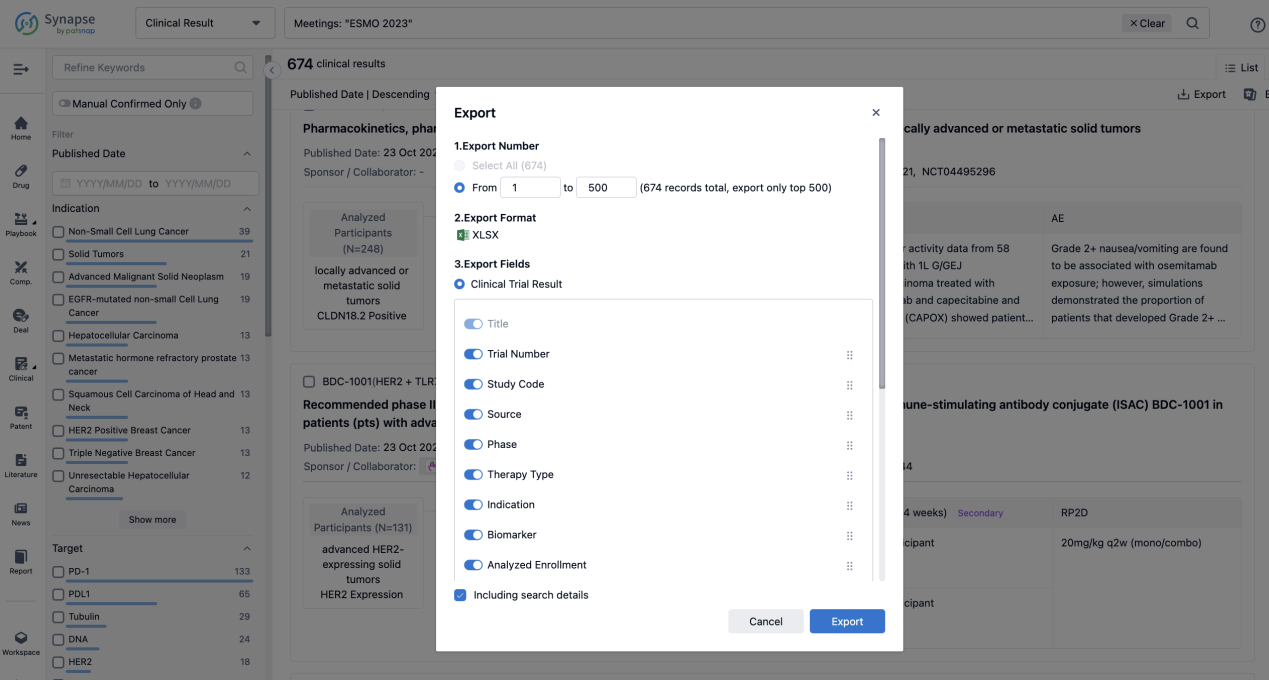
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!