The Discovery Process of TAK-279 and the Deep Logic Behind its Acquisition by Takeda
Recently, the top journal JMC updated an article, reviewing the discovery process of Nimbus company’s TYK2 inhibitor NDI-034858. This star molecule has also attracted much attention due to its significant deal with Takeda. On December 13, 2022, Takeda and Nimbus reached a $6 billion cooperation on the TYK2 allosteric inhibitor NDI-034858 (with an initial payment of $4 billion), which was renamed as TAK-279 after the acquisition.
Nimbus is a biopharmaceutical company established for 13 years, mainly using computer technology to expedite drug discovery and development. Since its inception, Nimbus has completed multiple rounds of financing, with Bill Gates, Eli Lilly Venture Fund, and Pfizer investing in multiple rounds. Schrödinger, a listed drug discovery company, is also an investor. Nimbus’s clinical stage projects include TYK2 and HPK1.
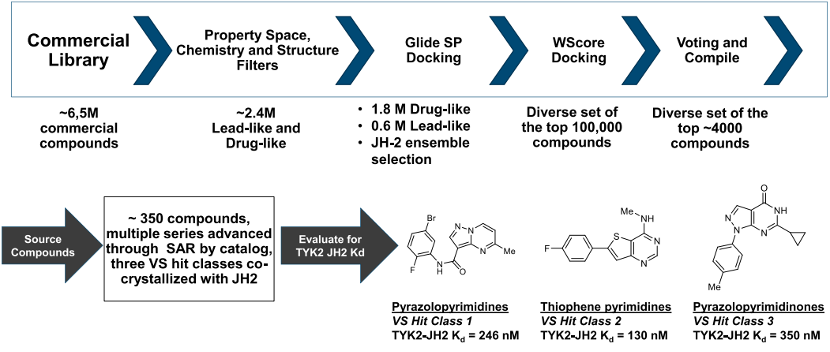
The article mentioned that virtual screening was conducted for the binding of compounds with the JH2 structural domain. Firstly, spatial attributes were restricted, and 2.4 million drug-like and lead-like molecules were screened out from 6.5 million commercial compounds. Then, based on the scoring process of binding with the JH2 structural domain, three parent nucleus fragments were finally screened out. The Kd of these three parent nucleus molecules binding with TYK2 JH2 domain ranges from 130nM to 350nM.
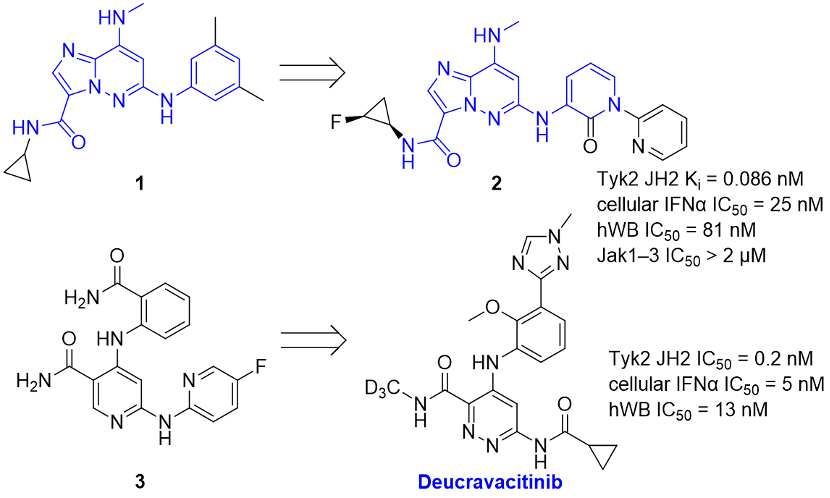
The Nimbus article mentioned the continued modification based on the Pyrazolopyrimidine core of hit class 1. Considering the modification from compound 1 to compound 2 disclosed by BMS in the article published in MedChemComm in December 2016, it is highly probable that Nimbus also referred to the successful experience of BMS in achieving high selectivity for JAK1-3 kinases by targeting the TYK2 JH2 domain and chose to develop based on the Pyrazolopyrimidine core of hit class 1. Subsequently, Nimbus used Free Energy Perturbation technology (FEP+) to calculate the binding force of ligands designed for the JH2 and JH1 binding sites of TYK2, while also considering the entropy and enthalpy effects of the ligands in solvents and proteins, as well as the local dynamics and second-shell effects of protein residues. Finally, after medicinal chemistry modifications and thorough exploration of Structure-Activity Relationship, the structure of candidate molecule NDI-034858 (TAK279) was determined.
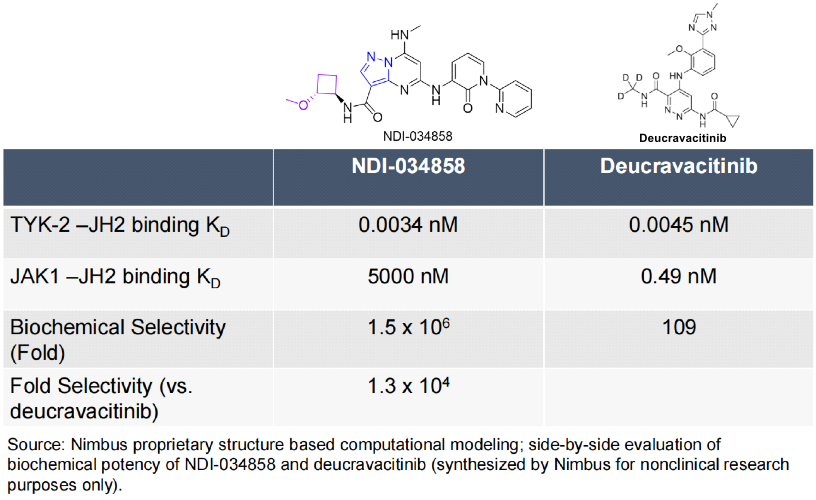
However, the approved dose for BMS's Deucravacitinib treatment of psoriasis is 6mg QD, whereas the estimated dose for TAK279 clinical treatment of psoriasis is over 30mg QD. Why does TAK279 have BIC potential? The following image is a comparison of in vitro selectivity posted in Nimbus Company's poster, intending to demonstrate that selectivity is a huge advantage, and high selectivity can avoid inhibition of JAK1-3, thus reducing potential side effects in patients and ultimately avoiding possible black box warnings from JAK inhibitors. In the image below, the Kd of NDI034858 binding to TYK2-JH2 is 0.0034nM, and the Kd of binding to JAK1-JH2 is 5000nM; the selectivity has reached 1.5 million times. The selectivity of Deucravacitinib is only 109 times, while the selectivity of TAK279 is 13,000 times that of Deucravacitinib.
However, in vitro selectivity cannot accurately assess safety risks. In the experiment of primary human peripheral blood mononuclear cells (PBMC) stimulated by IL-12, the cellular activity of the compound is measured by inhibiting the phosphorylation of STAT4. However, the in vitro test of TYK2-JH2 or JAK1-JH2 is just a segment of recombinant protein binding with the compound, while the real situation is the binding of the full-length TYK2-JH2 or JAK1-JH2 protein with the compound; cell ATP concentration related to the physiological condition also affects phosphorylation. All of these will affect the results of PBMC cell activity. In addition, the exposure amount of the compound in the body, the exposure amount under NOAEL, etc., will affect the safety window. The bioavailability of TAK279 in mice, rats, dogs, and monkeys is 70%/37%/47%/47% respectively, with hPPB Fu=23%, all of these could potentially lead to higher clinical doses of TAK279 compared to Deucravacitinib (psoriasis 30mg QD and above).
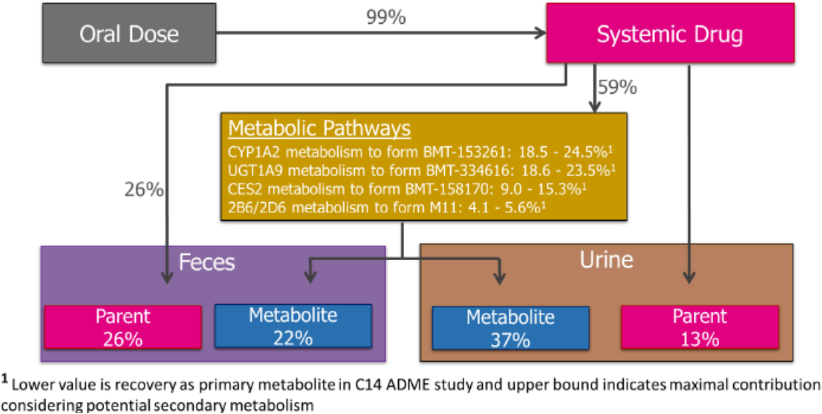
Deucravacitinib is quickly absorbed orally in animals (Tmax = 0.5 ~ 5 h). Despite being a substrate for extraintestinal efflux transporters P-gp and BCRP, it exhibits high absolute oral bioavailability in both animals and humans (99%), with a range of 87% to 100%. This indicates good absorption in animals and near-complete absorption in humans, suggesting that intestinal P-gp and/or BCRP do not limit its oral absorption. There is no significant gender difference, exposure loss, or accumulation after oral administration of deucravacitinib in mice, rats, rabbits, and monkeys. These are all excellent drug characteristics.
Deucravacitinib is metabolized in the body through four different pathways: CYP1A2-mediated N-demethylation on triazole to form BMT-153261, human carboxylesterase 2 (CES2)-mediated cyclopropyl acylamide hydrolysis to form BMT-158170, UGT1A9-mediated N-glucuronidation to form BMT-334616, and CYP2B6 and CYP2D6-mediated monooxygenation on deuterated methyl to form M11. All four of these biological transformation pathways are present in each species, so there is a similar spectrum of metabolites in all species, but the quantities vary. BMT-158170 and BMT-153261 are the major metabolites in human circulation.
BMT-153261 is an active metabolite, with its activity close to that of the parent drug, Deucravacitinib. In human clinical trials, the circulating exposure of BMT-153261 accounted for approximately 20% of the total exposure of drug-related components. This is one of the reasons for the low clinical dose of deucravacitinib: both the parent and metabolite are effective.
There is another question: Compound 2 is a compound in BMS's patent, its structure is very similar to that of NDI-034858, and its selectivity is significantly better than deucravacitinib. Why hasn't it been developed? Perhaps this is due to different understandings of the benefit-risk ratio between BMS and Nimbus company. From a later clinical perspective on the treatment of indications for psoriasis, the selectivity of deucravacitinib is sufficient. As for other indications, they await further observation.
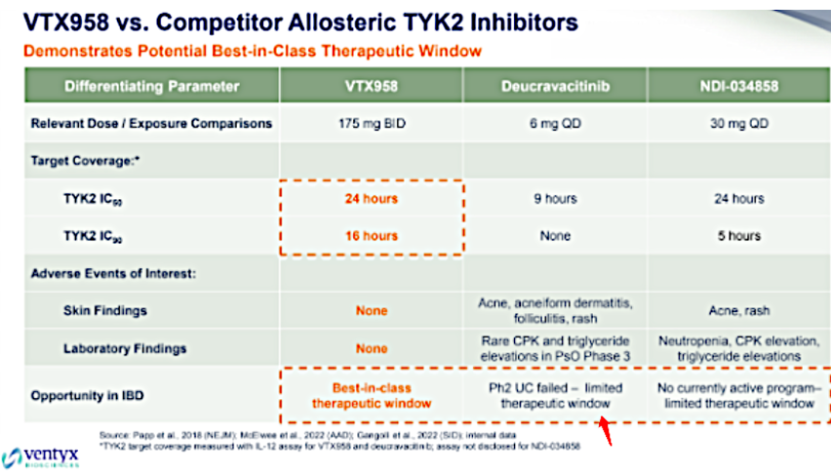
The above figure is a comparison of clinical doses and AEs, generated by Ventyx Company, to demonstrate that their molecule VTX958 is a BIC. Though deucravacitinib can only cover TYK2 IC50 for 9 hours at a 6mg qd dose and fails to cover TYK2 IC90, it might still be effective at low doses due to the active metabolite BMT-153261.
However, deucravacitinib is not perfect. Compared with placebo treatment, deucravacitinib treatment is associated with an increased incidence of asymptomatic creatine phosphokinase (CPK) elevation and rhabdomyolysis. Also, deucravacitinib treatment is associated with an elevation in triglycerides levels, the impact of this increased parameter on cardiovascular morbidity and mortality is yet to be determined. During the treatment phase, patients should have their serum triglycerides assessed regularly in accordance with hyperlipidemia clinical guidelines. Deucravacitinib treatment is also associated with an increase in liver enzyme levels compared to placebo treatment. Although there is preclinical basis for TYK2 inhibitor's high selectivity for JAK1-3, it is currently unclear if TYK2 inhibition might be associated with the observed or potential adverse reactions of JAK inhibition (thromboembolism, pulmonary embolism). Deucravacitinib has not yet been approved for RA.
According to a news release by BMS, an evaluation of deucravacitinib in a phase 2 study for moderate to severe ulcerative colitis (UC) did not meet the primary endpoint of clinical remission at week 12, nor did it meet its secondary efficacy endpoint. The failure of the phase 2 UC clinical trial could be due to the narrow therapeutic window, which might also be one of the reasons why BMS continues to roll out second-generation TYK2 inhibitors. TAK279 has conducted clinical trials for psoriasis and psoriatic arthritis, consistent with deucravacitinib, it has a risk of elevated CPK and triglycerides at 30mg QD, and current clinical data suggest a narrow therapeutic window.
In the non-head-to-head comparison of clinical effectiveness, in BMS's IM011-011 Phase II clinical trial, the proportion of patients achieving PASI 75 was 67%-75% in the patient group receiving a dose of 3 mg twice daily or higher of deucravacitinib; the PASI 90 reached 43%-44%, and for PASI 100 there were 9%-25% of patients.
In the phase II trials of Takeda's TAK-279, the percentage of patients in the TAK279 group reaching PASI 75 (at 44%, 68%, 67% respectively; 5 mg, 15 mg, 30 mg), achieving the primary study endpoint; the proportion of TAK-279 patients reaching PASI 90 was significantly higher (at 21%, 45%, 46% respectively; 5 mg, 15 mg, 30 mg), and the ratio reaching PASI 100 were 10%, 15%, 33% (5 mg, 15 mg, 30 mg).
In BMS' phase III clinical trials, at week 16, the proportion of Deucravacitinib (6mg) group achieving PASI 75 were 58.7% and 53.6%; the proportion reaching PASI 90 were 36% (POETYK PSO-1) and 27% (POETYK PSO-2).
Ignoring that the Takeda's clinical group had a lower proportion of patients with prior biological treatment (15% vs 43%), TAK-279's efficacy in psoriasis treatment was only nearly to that of BMS' phase II, and higher than BMS' phase III data.
The quality of decisions in drug development is far more important than speed and cost. BMS' high-throughput screening found an allosteric pocket that greatly improved the selectivity of TYK2 inhibitors for JAK1-3, while Nimbus' initial choice of TYK2's orthosteric pocket was not an ideal drug target. After referring to BMS' published MedChemComm in 2016, Nimbus made a breakthrough. Correct decisions must be based on a deep understanding of drug action mechanisms, translational medicine, and biology.
Looking forward, we are hopeful that Takeda's TAK-279 can realize its preclinical advantage of high selectivity in the clinic and truly earn its BIC title, and more importantly, benefit the patients.
Reference
1.Craig E. Masse et.al; Discovery of a Potent and Selective Tyrosine Kinase 2 Inhibitor: TAK-279.https://doi-org.libproxy1.nus.edu.sg/10.1021/acs.jmedchem.3c00600.
2.Nagasaka et al;Penetrating the central nervous system sanctuary of KRAS, a target once thought “undruggable”.Transl Lung Cancer Res 2023;12(4):665-668.
3.Sheng Jiang et al; Development and Therapeutic Implications of Tyrosine Kinase 2 Inhibitors,J. Med. Chem. 2023, 66, 4378−4416.




