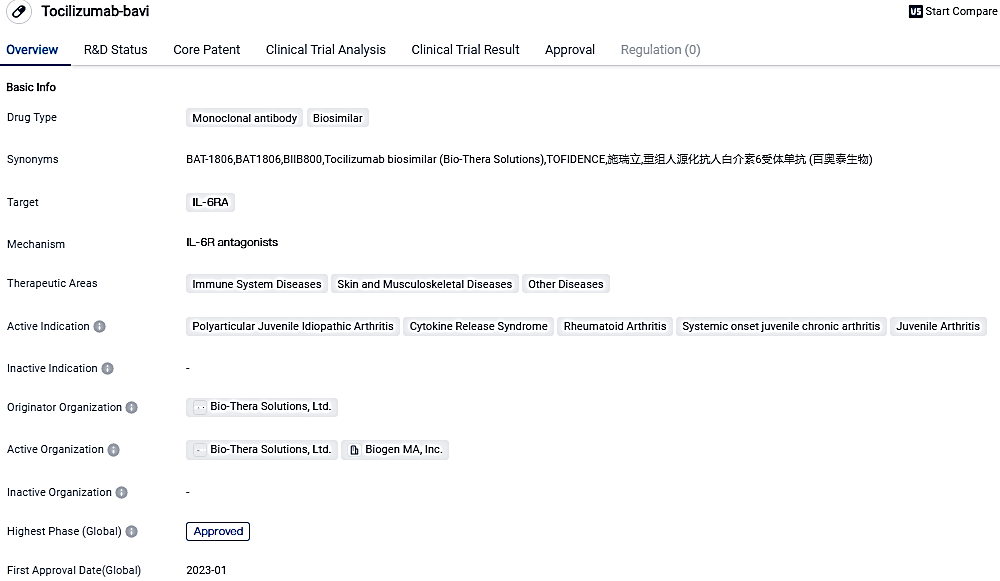
The FDA has granted approval for TOFIDENCE™ (tocilizumab-bavi), a biosimilar of ACTEMRA®, created by Bio-Thera Solutions
Bio-Thera Solutions, Ltd, a firm involved in the commercial production of biopharmaceuticals and the development of a succession of biosimilars and innovative products, has made it known that its associate company, Biogen, was recently notified by FDA about the approval of TOFIDENCE (tocilizumab-bavi) in an intravenous mixture. This is a biosimilar monoclonal antibody which refers to ACTEMRA.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The intravenous formula of TOFIDENCE has received authorization for use in treating moderately to severely active rheumatoid arthritis, polyarticular juvenile idiopathic arthritis and systemic juvenile idiopathic arthritis. This is Bio-Thera's inaugural FDA-accredited drug in the US, and the first biosimilar from a Chinese pharmaceutical firm to gain approval in the US.
Tocilizumab-bavi, a monoclonal antibody, binds to interlukin-6 receptors and is prescribed for various inflammatory autoimmune conditions, encompassing rheumatoid arthritis, polyarticular juvenile idiopathic arthritis and systemic juvenile idiopathic arthritis.
"Securing approval for TOFIDENCE signifies a pivotal milestone for Bio-Thera. It's our first product that has gained FDA approval in the United States." stated Bio-Thera CEO, Shengfeng Li. "Our company-focus is on creating biosimilars for global patients, and this approval is a testament to that commitment."
Biogen and Bio-Thera initiated a collaboration on TOFIDENCE (BAT1806/BIIB800) in April 2021. Developed by Bio-Thera, TOFIDENCE will be marketed by Biogen in all territories, excluding China (inclusive of Hong Kong, Macau, and Taiwan).
FDA's authorization of TOFIDENCE relied on comprehensive analytical, preclinical, and clinical data submitted by Biogen in September 2022. Extensive analysis of TOFIDENCE's structure, physicochemical properties, and biological attributes was accomplished, backing its biosimilarity with the reference drug.
Moreover, a double-blinded, randomized, single-dose, three-arm, phase I parallel study compared the pharmacokinetics, safety, and immunogenicity of TOFIDENCE with both the US and EU versions of reference tocilizumab in healthy volunteers. Also, a double-blind, randomized, multi-dose, phase III parallel study compared TOFIDENCE with tocilizumab to ascertain commensurate efficacy and similar pharmacokinetic, safety, and immunogenicity indications in rheumatoid arthritis patients that didn't respond well to methotrexate.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
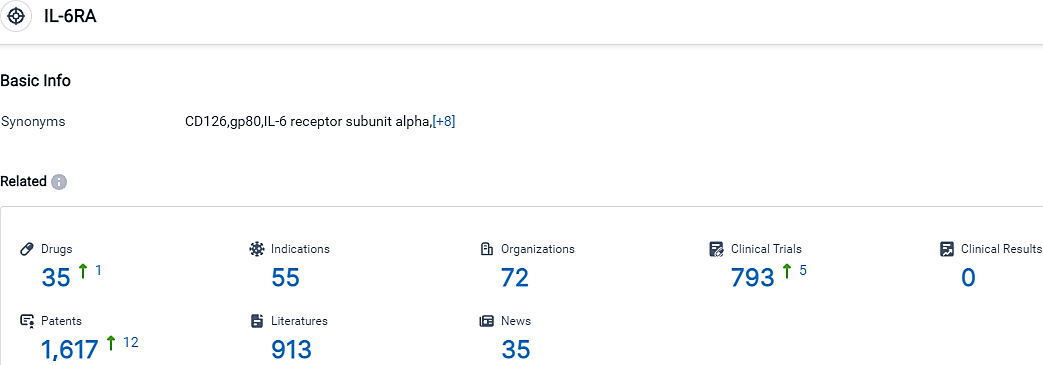
According to the data provided by the Synapse Database, As of October 17, 2023, there are 35 investigational drugs for the IL-6RA target, including 55 indications, 72 R&D institutions involved, with related clinical trials reaching 793,and as many as 1617 patents.
Tocilizumab-bavi holds potential in treating a range of immunological, dermatological, and musculoskeletal disorders, among others. Its authorization in China indicates compliance with essential regulatory norms, potentially offering a beneficial treatment alternative for patients. More investigation and evaluation are needed to determine its future influence and market prospects in the drug industry.