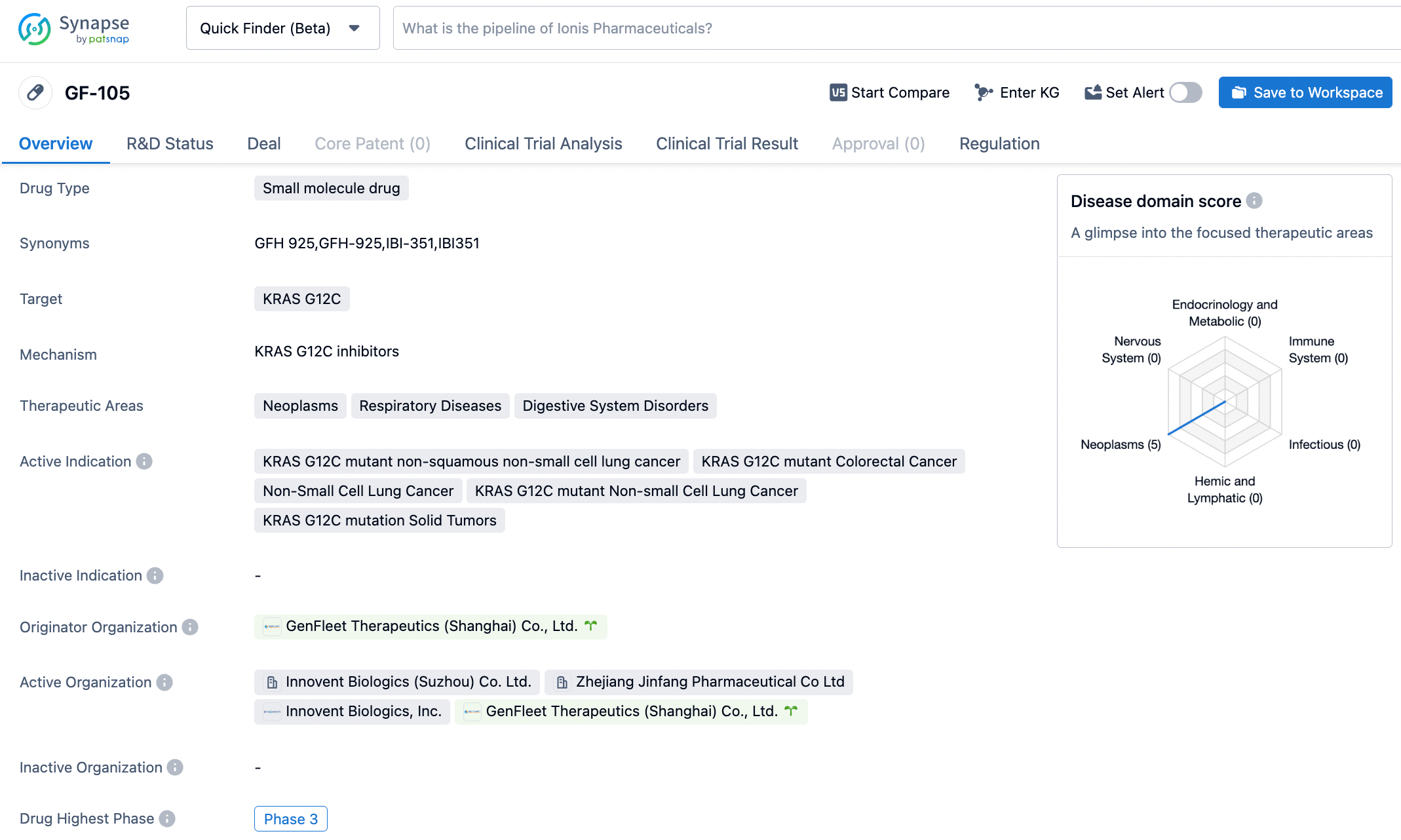
The KRAS G12C inhibitor IBI351 tablet by Innovent Biologics is set to be included in priority review for the treatment of advanced non-small cell lung cancer
On October 24, 2023, the latest announcement on the official website of the Drug Evaluation Center of the National Medical Products Administration (NMPA) in China reveals that the Category 1 new drug, GFH925 (IBI351), applied by Innovent Biologics, is set to be included in the priority review. This drug is intended for the treatment of advanced non-small cell lung cancer (NSCLC) with KRAS G12C mutations, which had received at least one systemic treatment.
GFH925 (IBI351) is a highly effective orally administered new molecular entity compound which has been independently developed by GenFleet Therapeutics, owning full intellectual property rights. This compound inhibits the KRAS protein by covalently and irreversibly modifying the cysteine residues of the KRAS G12C protein mutant, thereby downregulating its activation level. Additionally, GFH925, having demonstrated high selective inhibition efficacy in preclinical cysteine selectivity tests, after inhibiting the KRAS protein can further inhibit the downstream signal transduction pathway, induce tumor cell apoptosis and cell cycle arrest, achieving anti-tumor effects.
On September 2, 2021, GenFleet Therapeutics and Innovent Biologics announced a global exclusive licensing agreement, where Innovent Biologics, as the exclusive partnership, obtained the development and commercialization rights in China (including Mainland China, Hong Kong, Macau, and Taiwan) of GFH925 (KRAS G12C inhibitor), a candidate drug targeting the oncogenic driver gene KRAS G12C common in lung cancer and other solid tumors. They also have an option for global development and commercialization.
At the 2022 annual meeting of the Chinese Society of Clinical Oncology (CSCO), GenFleet Therapeutics announced new findings (NCT05005234) from its Phase Ia clinical study of GFH925 for the treatment of solid tumors and was presented orally. As of July 29, 2022, a total of 67 subjects with advanced malignant tumors that had failed or were intolerant to standard treatment were enrolled in this study, including 61 cases of non-small cell lung cancer, 5 cases of colorectal cancer, and 1 case of pancreatic cancer. Nearly 50% of the subjects had received second-line or higher treatment, and 37.7% of the lung cancer patients had baseline brain metastasis.
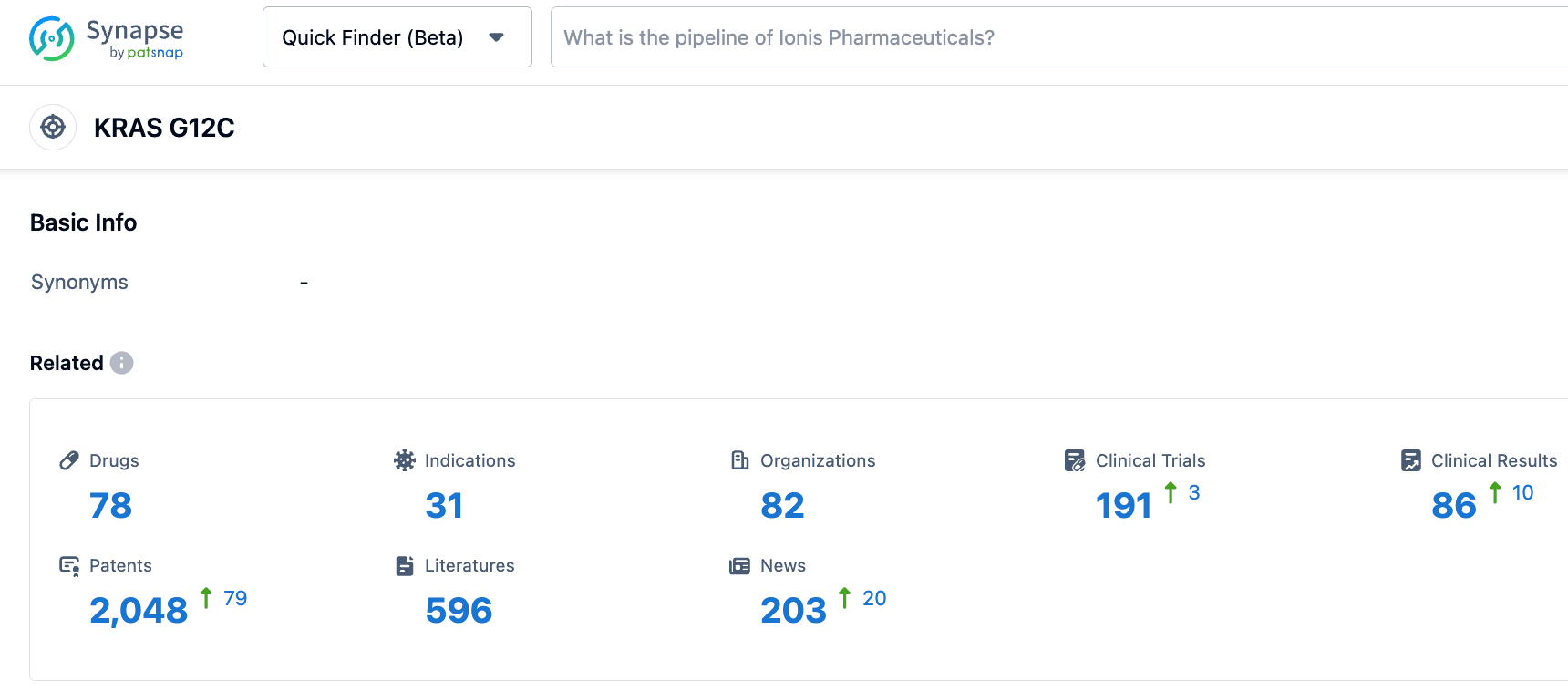
According to the Synapse database, as of October 25, 2023, there are 78 drugs under investigation targeting the KRAS G12C, intended for 31 indications, conducted by 82 institutions, involving 191 related clinical trials and as many as 2042 patents. The FDA has currently approved two KRAS G12C inhibitors, and many others are in clinical stages in China and abroad. The KRAS G12C, once thought to be "undruggable", has now become one of the hottest targets for drugability.