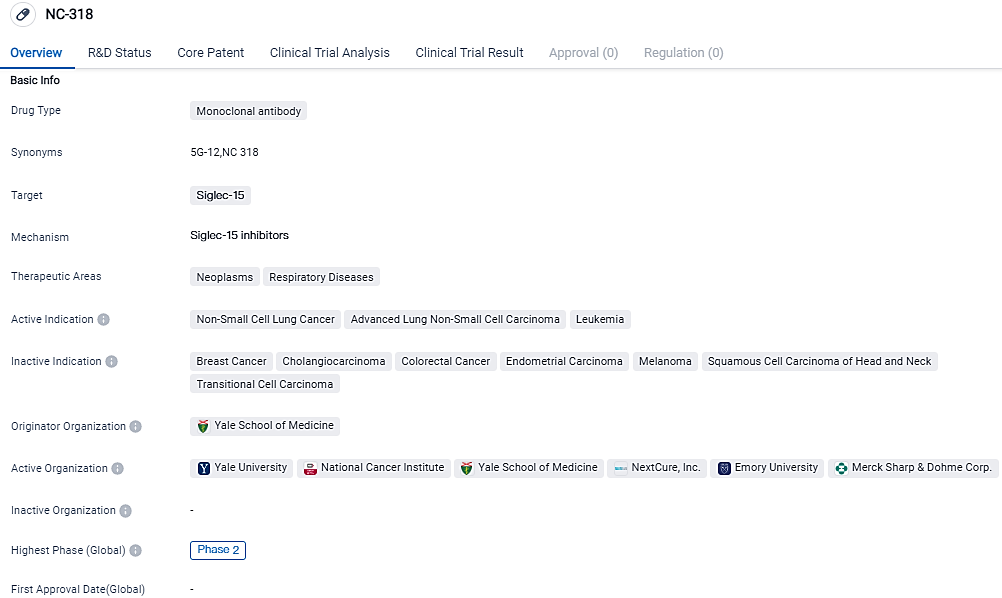
The NC318 Phase 2 trial, in Combination with Pembrolizumab, shows clinical activity in treating patients with advanced NSCLC
NextCure Inc., a biopharmaceutical corporation in the clinical phase, dedicated to the invention and progression of innovative, trailblazing immunomedicines for the treatment of cancer and other immunity-associated diseases, today made public the showcase of Phase 2 clinical study records by their partners at Yale Cancer Center. The data unveils a therapeutic advantage in patients suffering from advanced, non-responsive PD-1 axis inhibitor non-small cell lung cancer, who have been treated with a combined regimen inclusive of NC318, a Siglec-15 monoclonal antibody, and Pembrolizumab, an anti-PD-1 antibody.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
NC318, a humanized IgG1 mAb targeting S15, limits the interactions of S15 with both myeloid cells and T lymphocytes found within the tumor environment, thereby reducing immune inhibitory signals. Next Cure's earlier monotherapy study showed the potency of NC318 as a solitary agent in a Phase 1/2 dosage-escalation trial for individuals with advanced solid tumors.
The current ongoing study is a randomized test whose aim is to evaluate NC318's safety and effectiveness, either independently or paired with pembrolizumab. The examination of the effects of this combination in NSCLC subjects, who have shown disease progression while undergoing, or post, PD-1 axis inhibitor treatment, is the priority of this study portion.
The combination of NC318 and pembrolizumab shows promise, proving to be active against advanced PD-1 axis inhibitor resistant NSCLC, with 28% of patients showing a lasting clinical benefit.
Solomon Langermann, Ph.D., Chief Scientific Officer of NextCure, announced that, following these promising early findings, Yale continues to recruit participants to gather more evidence on NC318's clinical activity. "We anticipate the continuation of our mutually beneficial collaboration with Drs. Herbst and Gettinger and the Yale Cancer Center,” he noted.
Dr. Roy Herbst, the Ensign Professor of Medicine, expressed his excitement about their investigator-initiated trial as part of the NCI Lung SPORE, noting it had offered fresh perspectives on immune therapy resistance and its treatment, “We are enthusiastic about releasing additional data in the future."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
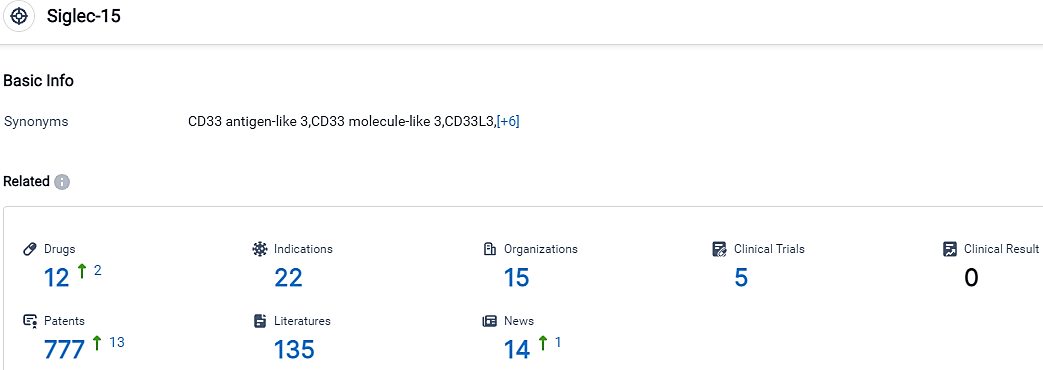
According to the data provided by the Synapse Database, As of September 18, 2023, there are 12 investigational drugs for the Siglec-15 target, including 22 indications, 15 R&D institutions involved, with related clinical trials reaching 5,and as many as 777 patents.
Non small cell lung cancer (NSCLC) is the most common type of lung cancer, accounting for 85% of all lung cancers. Therefore, the NSCLC drug market is very large and rapidly growing. According to a report by Grand View Research, it is expected that the global NSCLC drug market will reach $41.3 billion by 2025. Currently, some of the main NSCLC drugs include Opdivo, Keytruda, Tagriso, Alecensa, and Avastin. These drugs can be classified into different categories based on their working methods, such as immunotherapy, targeted therapy, and chemotherapy. In addition, many companies are developing new NSCLC drugs and therapies. Overall, the market for non-small cell lung cancer drugs is rapidly developing, and the research and approval of new drugs, as well as the pursuit of personalized treatment, all indicate that the market size will continue to grow.