The Partial Clinical Hold on BNT326/YL202 Has Been Lifted
The U.S. Food and Drug Administration (FDA) has removed the partial clinical hold previously imposed on MediLink Therapeutics' (Suzhou) Co., Ltd. Phase I trial, which is assessing BNT326/YL202 (NCT05653752) and was disclosed on June 17, 2024. The full response, which includes data analysis, an updated investigator brochure, informed consent forms for patients, and a revised clinical trial protocol, complies with the FDA’s demands by integrating enhanced risk mitigation strategies.
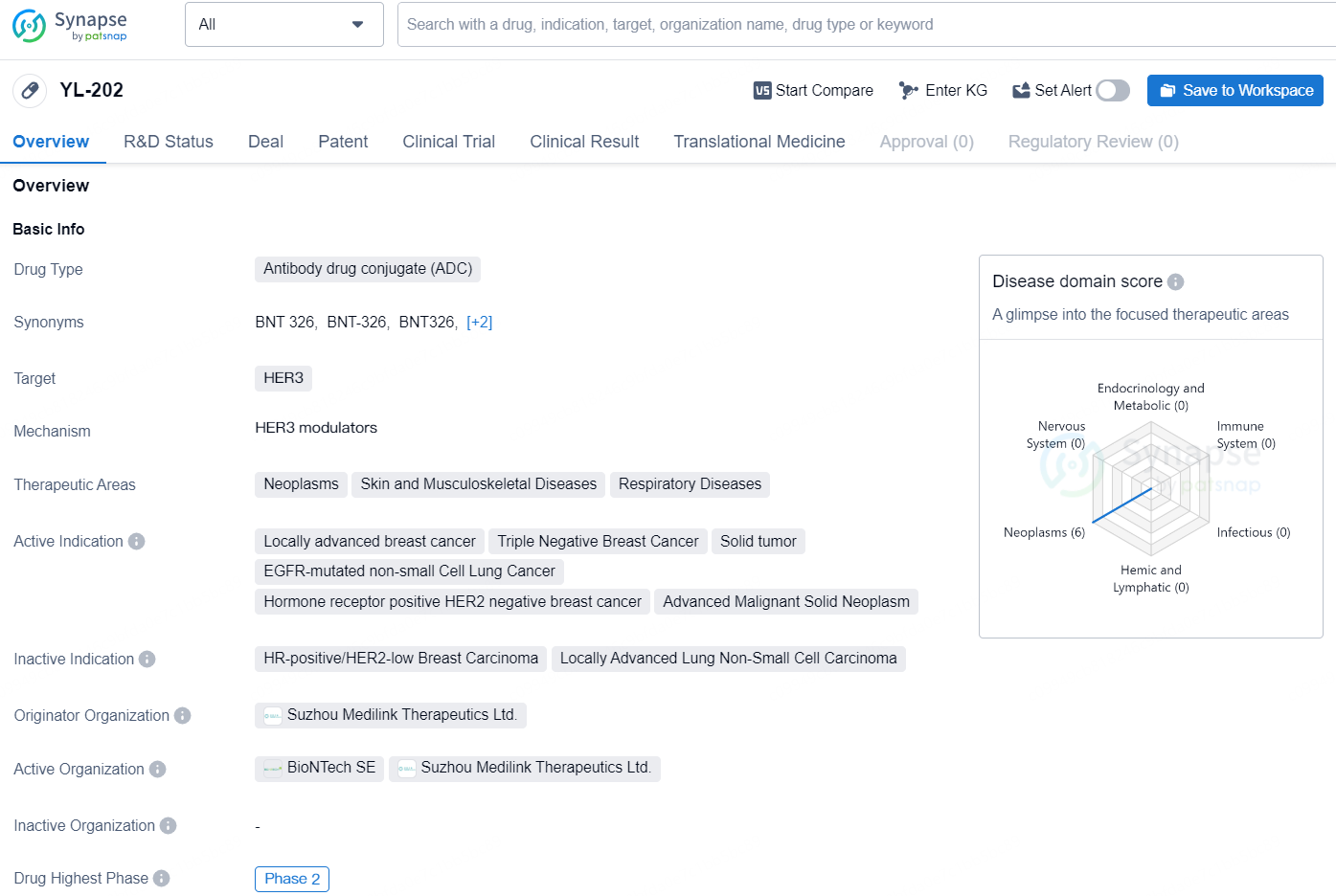
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
BNT326/YL202 is a promising ADC (antibody-drug conjugate) aimed at targeting Human Epidermal Growth Factor Receptor 3 (HER3). This candidate is under development through a partnership between BioNTech SE and MediLink. Patient recruitment for the clinical trial will resume with a focus on maintaining doses at or below 3 mg/kg, where the safety profile was manageable and there were signs of encouraging clinical activity.
MediLink, the clinical trial sponsor, noted a dose-dependent trend in treatment-related adverse events (TRAEs) for BNT326/YL202, including reduced neutrophil counts (neutropenia) and an uptick in mucositis cases. Such adverse events are frequently observed with established chemotherapy treatments and can elevate the risk of serious infections. Neutropenia is generally managed by reducing or interrupting doses, and/or administering primary prophylaxis, such as recombinant granulocyte colony-stimulating factors (G-CSFs), tailored to the patient's febrile neutropenia risk and the chemotherapy regimen.
Responding to safety data from the ongoing BNT326/YL202 trials, the companies quickly took precautionary steps, including halting new enrollments for dose levels above 3 mg/kg and lowering doses for existing participants in higher dose cohorts. Concurrently, MediLink informed the FDA and collaborated with BioNTech on analyzing the developing safety data and implementing additional risk mitigation strategies. These actions include updating the investigator brochure, patient informed consent forms, and clinical trial protocol to incorporate revised guidelines on dose delay, reduction, modification, and prophylactic measures for managing TRAEs.
Both BioNTech and MediLink remain committed to ensuring patient safety while continuing the development of BNT326/YL202 for treating solid tumors with significant unmet medical need.
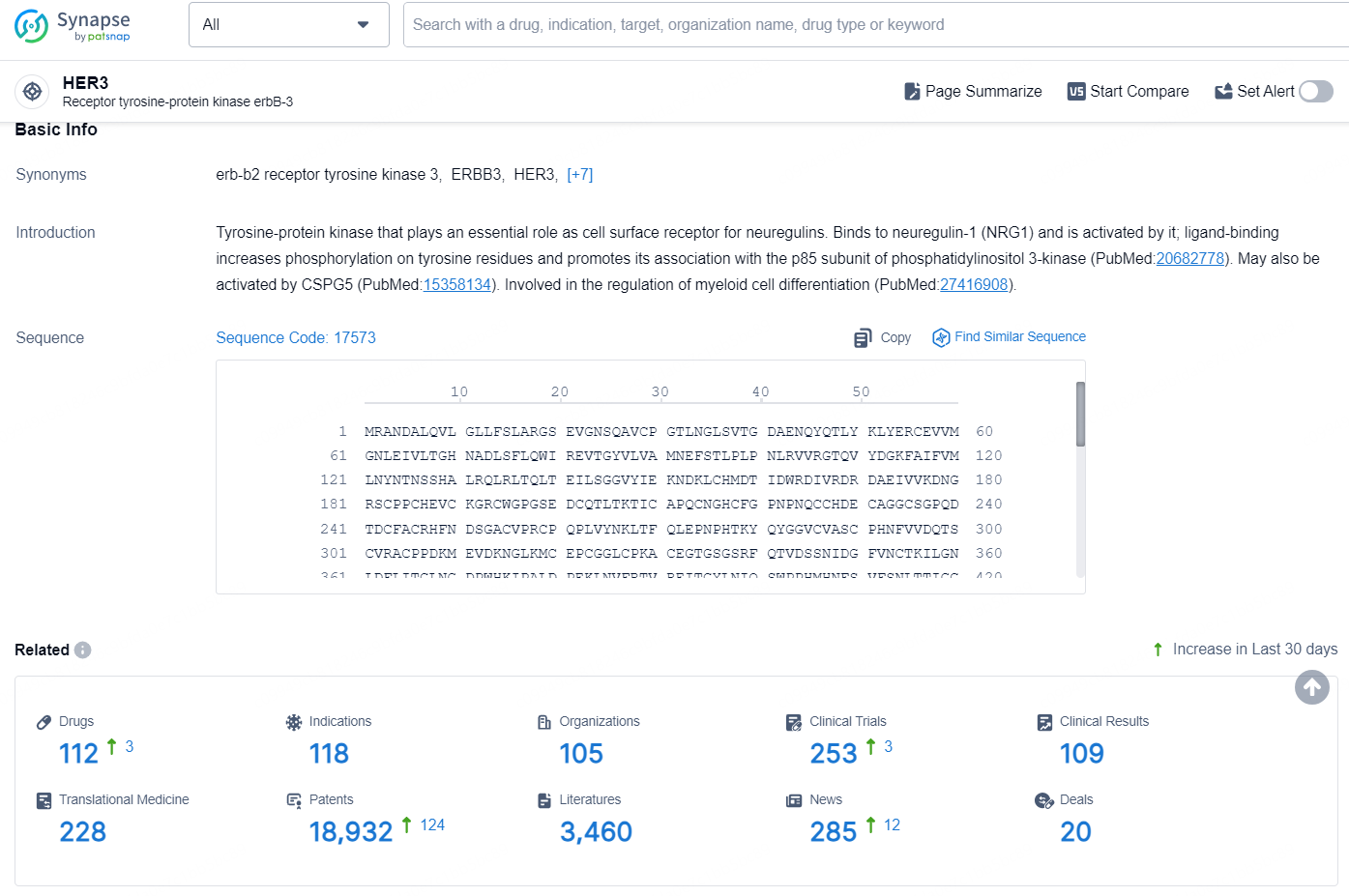
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of August 22, 2024, there are 112 investigational drugs for the HER3 targets, including 118 indications, 105 R&D institutions involved, with related clinical trials reaching 253, and as many as 18932 patents.
The drug YL-202 is an antibody drug conjugate (ADC) that targets the HER3 protein. It is being developed to treat a variety of therapeutic areas, including neoplasms, skin and musculoskeletal diseases, and respiratory diseases. The drug is currently indicated for locally advanced breast cancer, triple negative breast cancer, solid tumors, EGFR-mutated non-small cell lung cancer, hormone receptor positive HER2 negative breast cancer, and advanced malignant solid neoplasms.