The US FDA has given approval to Dong-A ST’s IMULDOSA™ (ustekinumab-srlf), a biosimilar for STELARA®
Dong-A ST (led by President/CEO Jae-Hun Jung, KRX: 170900) declared that its biosimilar Imuldosa™ (ustekinumab-srlf/DMB-3115), which references Stelara®, has received approval from the U.S. Food and Drug Administration (FDA). This marks the company’s second biosimilar to gain FDA approval following Sivextro® (tedizolid phosphate) in 2014, further showcasing its research and development capabilities.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The announcement follows the FDA's approval of the biologics license application (BLA) from Accord BioPharma, a fully owned division of Intas Pharmaceuticals, submitted in October 2023.
Imuldosa is a biosimilar to Stelara, a leading medication produced by Janssen Biotech Inc. for treating various autoimmune conditions, including plaque psoriasis, psoriatic arthritis, Crohn’s disease, and ulcerative colitis. This drug is among the highest-earning biopharmaceuticals, generating $10.86 billion in worldwide sales (as reported by IQVIA in 2023).
Joint development efforts for Imuldosa commenced in 2013 between Dong-A Socio Holdings and Meiji Seika Pharma. In July 2020, the rights for research and development and commercialization were transferred from Dong-A Socio Holdings to Dong-A ST to streamline project management. In July 2021, a global licensing agreement was established between Dong-A ST, Meiji Seika Pharma, and Intas Pharmaceuticals, which plans to market the biosimilar through its global branches, including Accord BioPharma in the U.S. and Accord Healthcare in the EU, UK, and Canada.
Furthermore, Accord Healthcare submitted a marketing authorization application (MAA) to the European Medicines Agency (EMA) in June 2023, which received acceptance in July 2023.
Dr. Jae-Hong Park, the R&D Head at Dong-A ST, stated, “This FDA approval signifies global recognition of Dong-A ST’s excellence in R&D and its competitive edge on the world stage. We anticipate a successful introduction of Imuldosa in the U.S., the largest pharmaceutical market globally, as we work towards developing innovative treatments to enhance our international presence.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
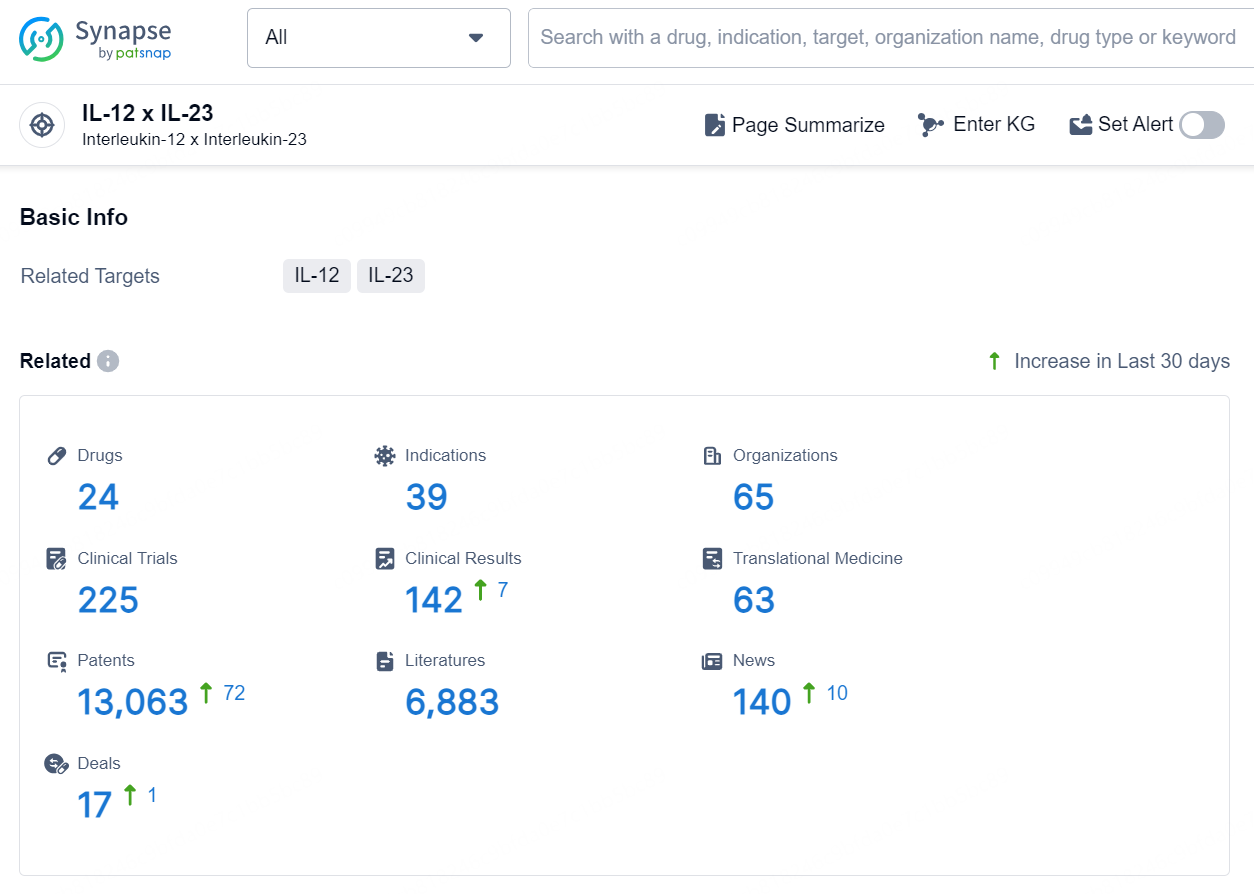
According to the data provided by the Synapse Database, As of October 15, 2024, there are 24 investigational drug for the IL-12 and IL-23 targets, including 39 indications, 65 R&D institutions involved, with related clinical trials reaching 225, and as many as 13063 patents.
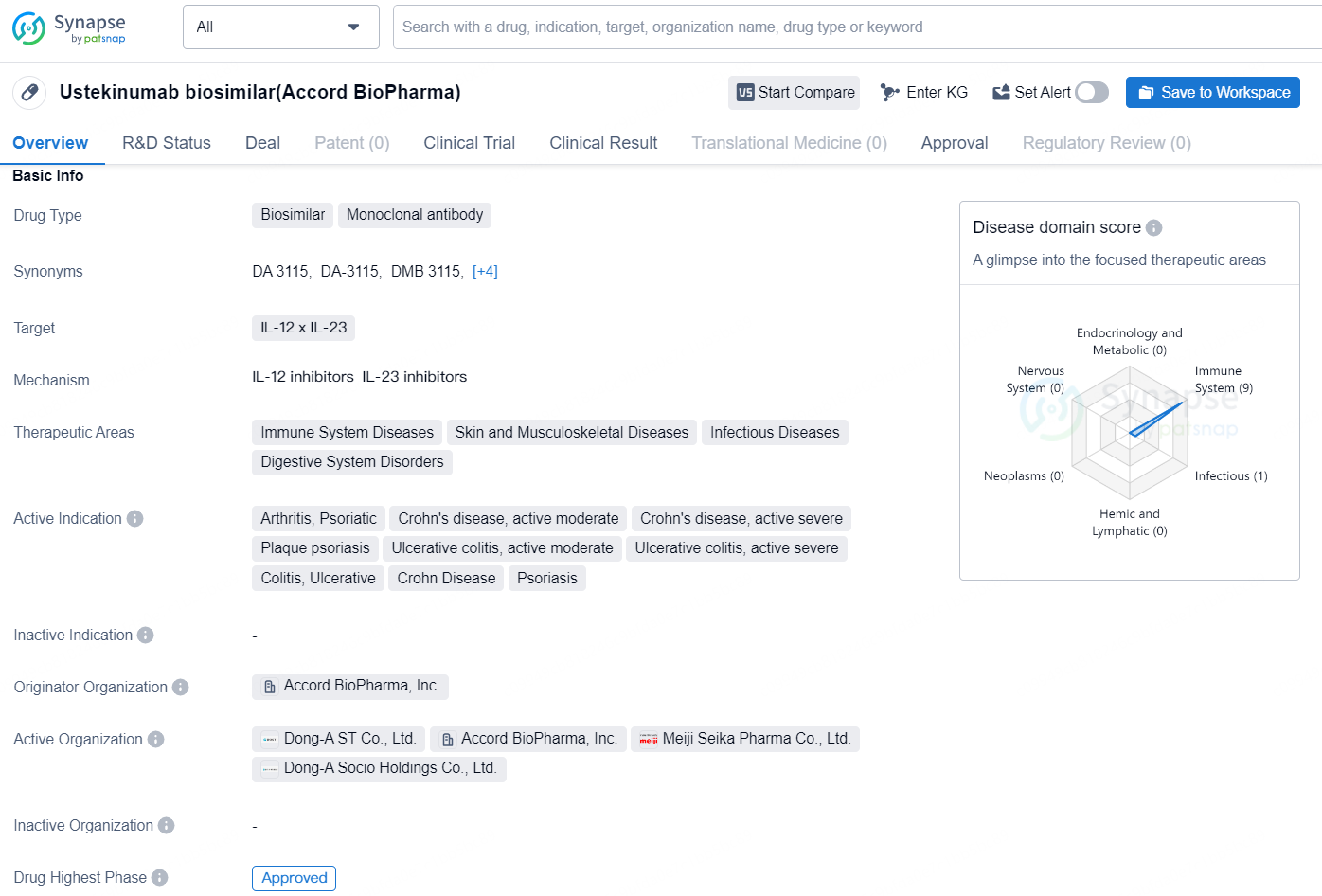
The Ustekinumab biosimilar (Accord BioPharma) is a monoclonal antibody drug type that targets IL-12 and IL-23. It is indicated for a broad range of therapeutic areas, including immune system diseases, skin and musculoskeletal diseases, infectious diseases, and digestive system disorders. The drug is approved for the treatment of arthritis, psoriatic arthritis, Crohn's disease (both moderate and severe), plaque psoriasis, ulcerative colitis (both moderate and severe), and colitis ulcerative.