TOLREMO Begins Phase 1 Trial of TT125-802, a Novel Drug Against Cancer Resistance
TOLREMO therapeutics AG has made an announcement that initiation of patient administration has occurred in their inaugural human clinical study. This study is designed to assess the safety profile, dose absorption and distribution, and drug effect mechanisms of their principal compound, TT125-802. The target demographic for this trial includes individuals diagnosed with various solid tumor types.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
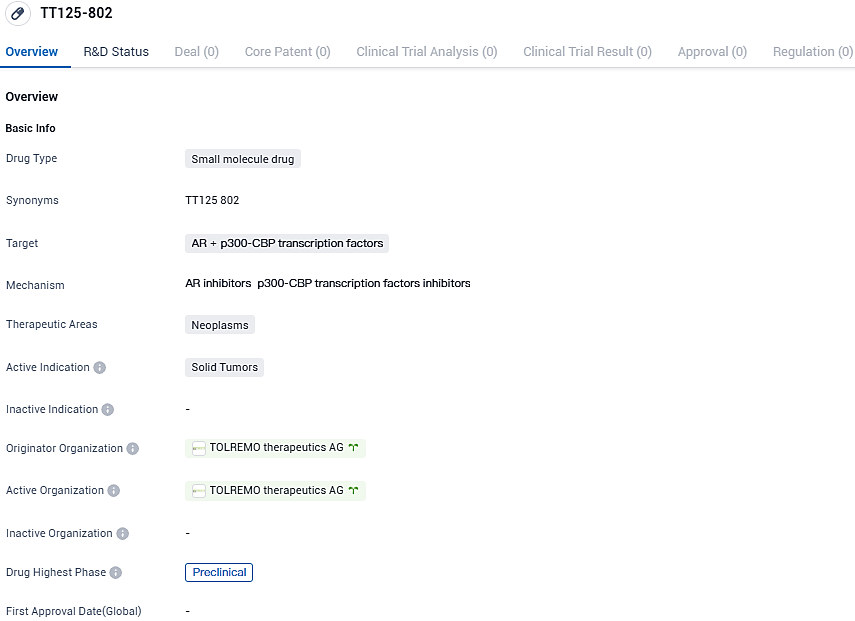
TT125-802 is a synthetically produced, ingestible compound designed as a CBP/p300 bromodomain antagonist. Its primary function is to deter cancer cells from developing resistance to drugs, thereby enhancing the effective duration and overall success of precise oncologic treatments. During experimental phases, this agent has shown efficacy in impeding key transcriptional circuits that facilitate the initial defiance of cancer cells against precise treatments.
"By capitalizing on our research acumen, we're cultivating a potent CBP/p300 blockade with the intent to counteract resilient tendencies in a wide gamut of present and envisaged oncologic therapies," remarked Stefanie Flückiger-Mangual, PhD, co-creator and CEO of the bioscience firm TOLREMO. "Our goal is resolute—tackling cancer resistance that arises at the transcriptional level to improve patient outcomes. The initiation of this clinical trial signifies a pivotal juncture for our institution."
The initial segment of the Phase I trial, aimed at assessing escalating doses, will encompass participants with various solid neoplasms. The foremost aim is to appraise the tolerability of TT125-802 when used in isolation. Further targets of this study will involve determining the compound's effect on biological processes, tracking its absorption and action within the body, ensuring it connects with its intended target, discerning the optimal dosage for continued research, and recognizing potential biomarkers to refine patient selection in subsequent stages.
Commencing in European medical facilities, with an eye towards expanding to American localities pending an affirmative Investigational New Drug (IND) review by the FDA, TOLREMO plans to meticulously progress TT125-802's clinical journey. This will eventually lead to investigations in concert with established targeted agents that inhibit molecular targets like KRAS, EGFR, or AR, particularly within advanced solid tumor classifications.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
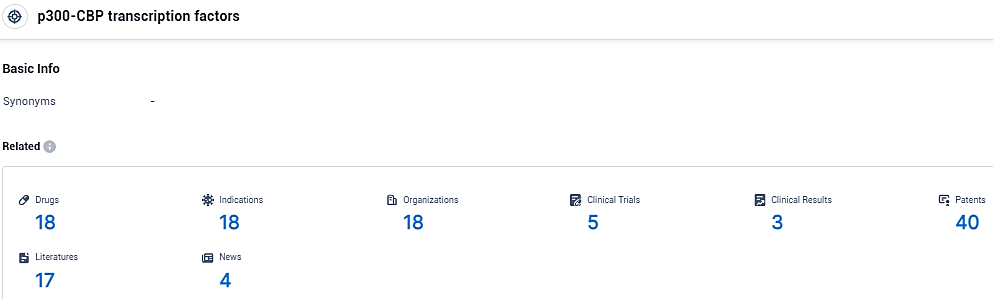
According to the data provided by the Synapse Database, As of December 5, 2023, there are 18 investigational drugs for the p300-CBP transcription factors target, including 18 indications, 18 R&D institutions involved, with related clinical trials reaching 5, and as many as 40 patents.
TT125-802 targets AR and p300-CBP transcription factors and aims to treat solid tumors, specifically in the neoplasms therapeutic area. Currently in the preclinical phase, further research and clinical trials are needed to evaluate its potential as a treatment option for patients with solid tumors.