Top 50 Pharmaceutical Companies R&D Progress: An Overview of Alexion 's 88 Drug Pipelines
Alexion Pharmaceuticals, Inc. is a biopharmaceutical company that was founded in 1992 and is headquartered in Connecticut, United States. The company focuses on the development and commercialization of therapies for rare diseases, with a particular emphasis on disorders of the immune system.
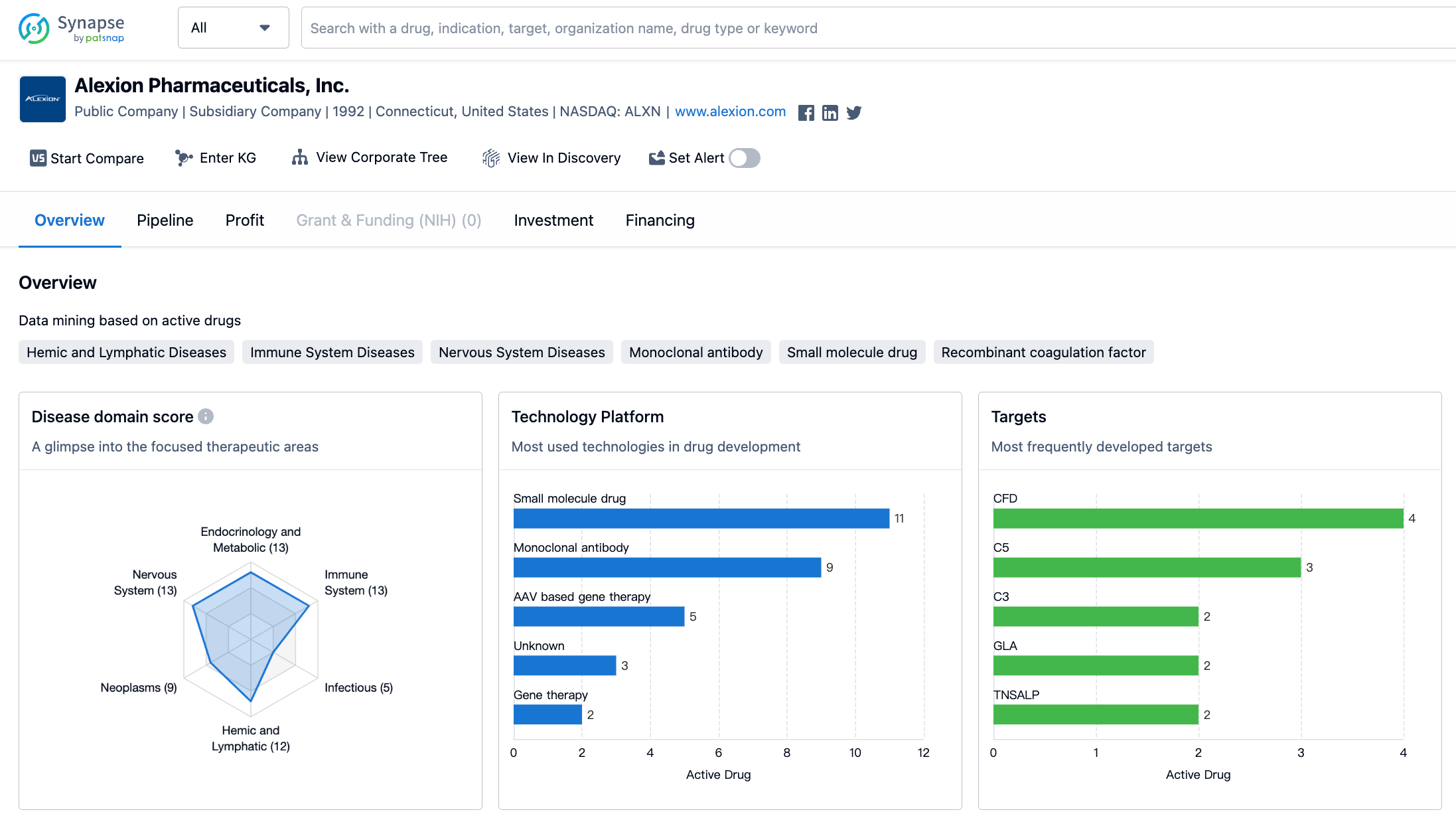
In terms of therapeutic areas, Alexion Pharmaceuticals has a diverse portfolio of drugs targeting various disease categories. The company has the highest number of drugs in the field of congenital disorders, with a total of 15 drugs. This is followed closely by nervous system diseases, immune system diseases, and endocrinology and metabolic diseases, each with 13 drugs. Hemic and lymphatic diseases, neoplasms, and other diseases have 12, 9, and 9 drugs respectively. The remaining therapeutic areas have a lower number of drugs, with otorhinolaryngologic diseases having the lowest count of only 1 drug.
When it comes to the most frequently developed targets by Alexion Pharmaceuticals, the company has focused on a range of proteins and enzymes. The most commonly targeted protein is CFD, with 4 drugs developed against it. This is followed by C5, C3, GLA, TNSALP, and factor Xa, each with 2 drugs. Other targets such as TTR, HS, MUT, LIPA, C5a, NS3/NS4A, ALK2, C5 + albumin, FcRn, SOD1, NS5A, C6 + complement system proteins, and CD200 have 1 drug developed against them.
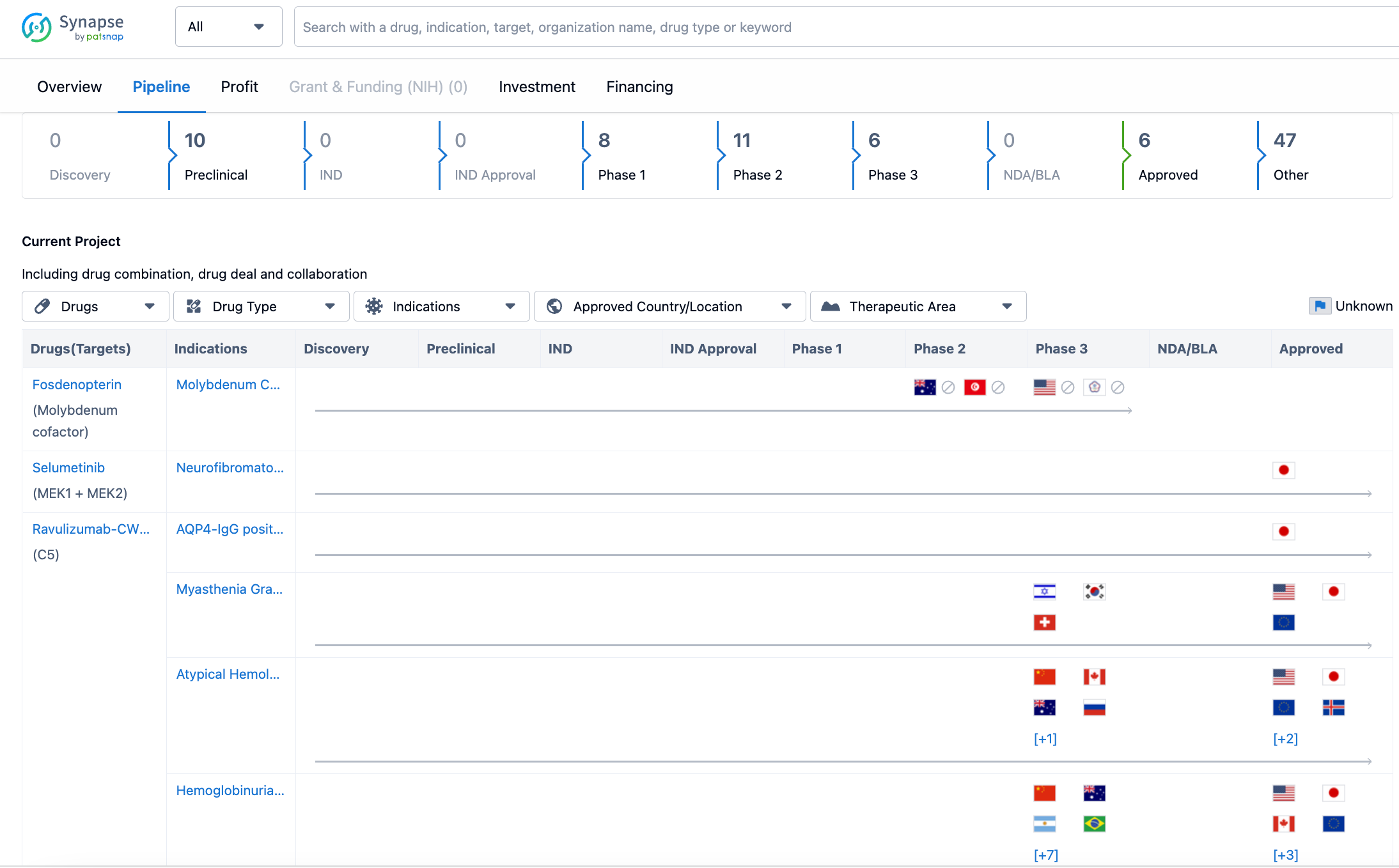
In terms of the pipeline, Alexion Pharmaceuticals has a number of drugs at different stages of development. As of the latest update on July 21, 2023, the company has 10 drugs in the preclinical stage, indicating that they are still undergoing testing in laboratory and animal models. There are no drugs in the discovery stage, suggesting that the company is not actively pursuing new drug targets at the moment. Additionally, there are no drugs in the IND (Investigational New Drug) stage or IND approval stage, which means that the company has not yet submitted these drugs for regulatory approval.
Moving further along the pipeline, Alexion Pharmaceuticals has 8 drugs in Phase 1, indicating that they have progressed to testing in human subjects for the first time. The company has 11 drugs in Phase 2, which means that these drugs are being tested for their efficacy and safety in a larger group of patients. In Phase 3, which is the final stage of clinical development before regulatory submission, Alexion Pharmaceuticals has 6 drugs. This suggests that the company is close to seeking approval for these drugs.
However, it is important to note that as of the latest update, there are no drugs in the NDA/BLA (New Drug Application/Biologics License Application) stage, which is the stage where the company submits the necessary documentation to regulatory authorities for approval. This indicates that Alexion Pharmaceuticals has not yet reached this stage with any of its drugs.
On the other hand, the company has 6 drugs that have already been approved. These drugs have successfully completed the clinical development process and have received regulatory approval for commercialization. It is worth mentioning that the majority of drugs in the pipeline, 47 in total, are categorized as "Other." This category likely includes drugs at various stages of development, such as early preclinical studies or late-stage clinical trials.
In summary, Alexion Pharmaceuticals, Inc. is a biopharmaceutical company that specializes in the development and commercialization of therapies for rare diseases, particularly those affecting the immune system. The company has a diverse portfolio of drugs targeting various therapeutic areas, with a focus on congenital disorders, nervous system diseases, immune system diseases, and endocrinology and metabolic diseases. Alexion Pharmaceuticals has also developed drugs targeting a range of proteins and enzymes, with CFD being the most frequently targeted. The company has a pipeline of drugs at different stages of development, with a significant number in the preclinical and clinical stages. However, as of the latest update, there are no drugs in the NDA/BLA stage, indicating that the company has not yet submitted any drugs for regulatory approval.