UK Regulators Greenlight Ariceum's Early Trial for I-123 Tagged PARP Blocker in Recurrent Glioblastoma Patients
Ariceum Therapeutics, an independent firm specializing in the biotechnology sector, has recently developed innovative radiopharmaceuticals intended for the precise detection and effective therapy of particularly resistant oncological diseases. The company is delighted to share the news that the UK Medicines and Healthcare products Regulatory Agency has granted them the green light to progress with a Phase 1 clinical study. This trial will assess 123I-ATT001, the company's proprietary drug marked with Iodine-123, designed to inhibit PARP, in individuals dealing with relapses of glioblastoma.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Following Ariceum's proposal for a Clinical Trial Authorisation (CTA) to the MHRA in the final month of 2023, approval has been granted, and the initiation of the company's Phase 1 trial is on track for mid-2024 in the United Kingdom. Ariceum pioneers as the initial firm undertakes a clinical study into Auger therapy much-needed for the treatment of recurrent glioblastoma—a notoriously challenging brain cancer variant.
Ariceum Therapeutics' CEO, Manfred Rüdiger, expressed his sentiments, “Securing the green light for a CTA from MHRA just a brief period post-proposal is a significant milestone for Ariceum. Given the bleak survival outlook tied to glioblastoma, which tops the list of adult brain malignancies in both occurrence and malignancy, and with no existing cure, we are eager to be at the forefront with Auger therapy clinical trials for this disease. Our broader vision includes assessing the potential for Auger therapy across various solid tumour profiles."
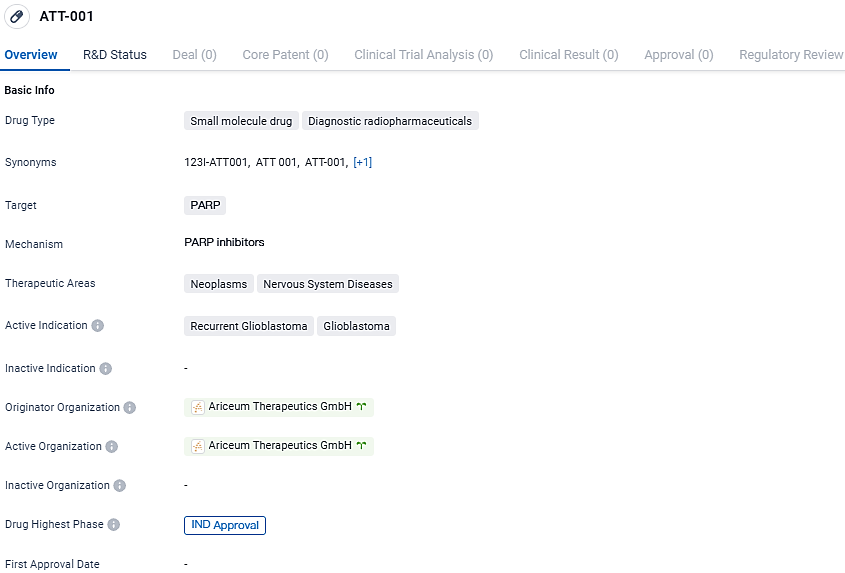
ATT001 is remarkable in its selective delivery of the radioactive component, Iodine-123, specifically targeting cancer cells that exploit the enzyme PARP for DNA repair mechanisms. The emitted Auger electrons from Iodine-123 possess low energy levels and confer their potent effects over concise distances, ideally calibrated to annihilate cancer cells while conserving surrounding healthy tissues.
An added advantage of utilizing Iodine-123 lies in its greater accessibility due to production in conventional cyclotrons. Ariceum concurrently delves into the application of 123I-ATT001 for additional solid tumour types, banking on the established targeting of PARP, which is prevalent across a spectrum of other cancerous afflictions.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
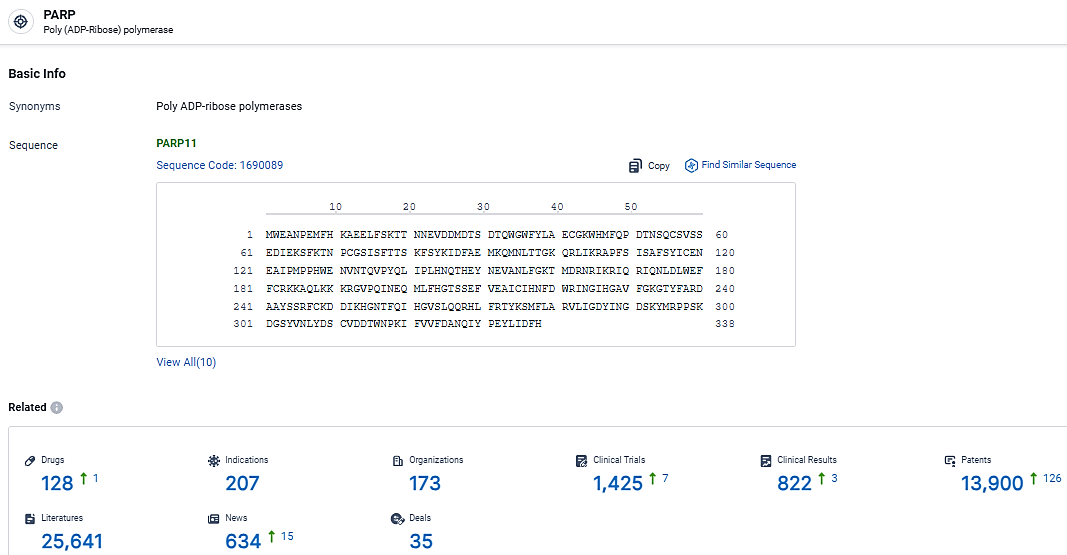
According to the data provided by the Synapse Database, As of March 5, 2024, there are 128 investigational drugs for the PARP target, including 207 indications, 173 R&D institutions involved, with related clinical trials reaching 1425, and as many as 13900 patents.
ATT-001 is indicated for the treatment of recurrent glioblastoma and glioblastoma, both of which are neoplasms affecting the nervous system. Ariceum Therapeutics GmbH is the originator organization, and the drug has reached the highest phase of development with IND approval.