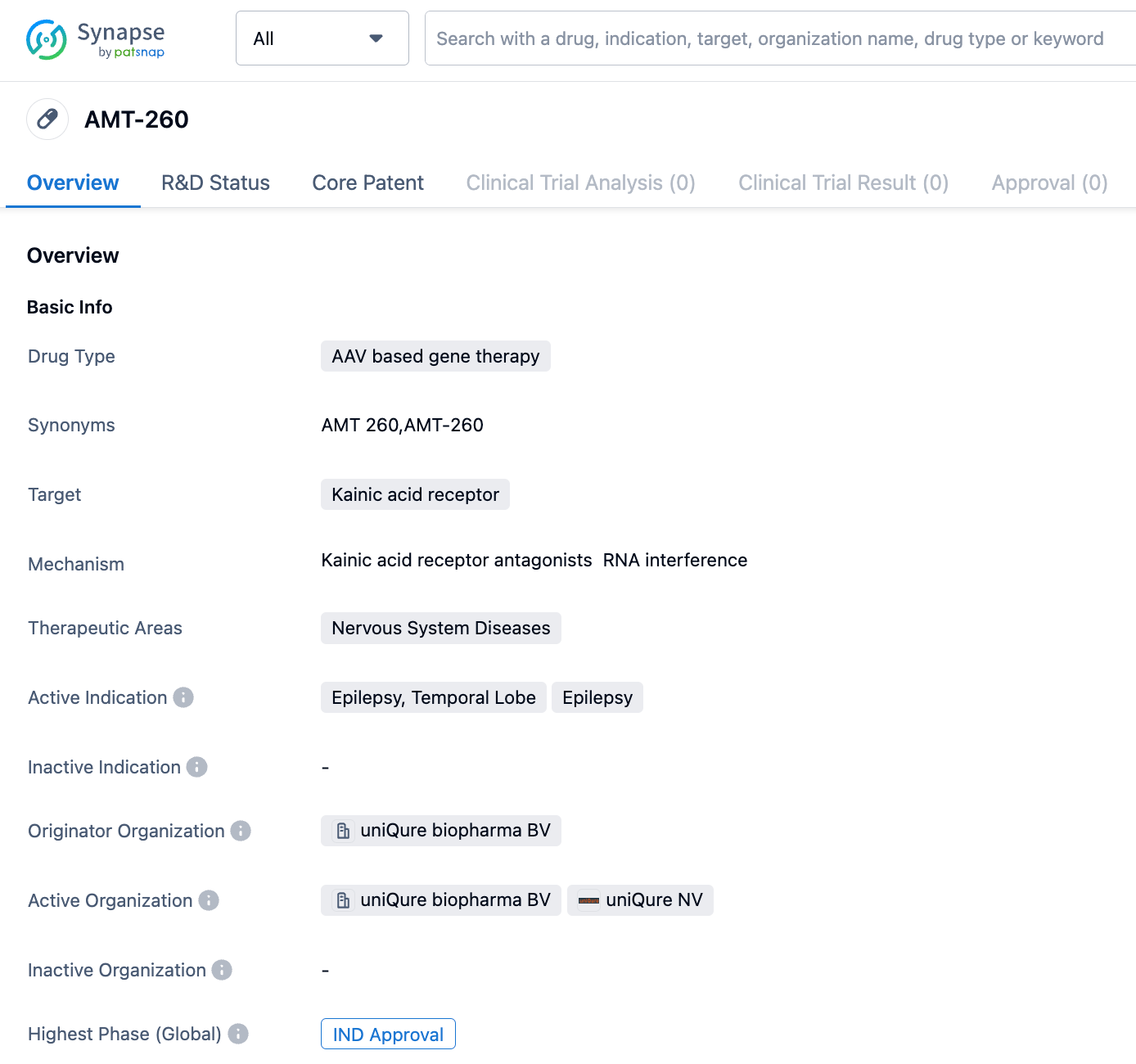
uniQure reveals that the FDA has approved the IND Application for their AMT-260 Gene Therapy, made to treat Refractory Mesial Temporal Lobe Epilepsy
uniQureN.V.,announces that their AMT-260 Gene Therapy candidate, developed for the treatment of Refractory Mesial Temporal Lobe Epilepsy, has received approval for the Investigational New Drug Application from the FDA.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The AMT-260 consists of an AAV9 vector that locally transmits two manufactured miRNAs to degrade the GRIK2 gene and lessen the unusual production of the GLUK2 subtype of the glutamate receptor believed to cause seizures in those with stubborn MTLE.
Walid Abi-Saab, uniQure's chief medical officer, announced, "Securing the IND for AMT-260 is a significant stride in pushing forward our pipeline and represents our subsequent scheme to initiate clinical trials in a field of considerable unsatisfied medical need. We recognize that treatment choices for patients suffering from stubborn MTLE are limited, and we are enthusiastic about commencing the clinical examination of this single-dose gene therapy approach as a plausible new cure.”
The premiere Phase I/IIa clinical trial will be carried out in the US and will be divided into two sections. Part one will include a multicenter, open-label trial that will divide patients into two dosing groups of six to test the safety, tolerability, and preliminary efficacy signs of AMT-260 in patients with stubborn MTLE. The second part is intended to be a randomized, managed trial aimed at producing confirmation of concept data.
Temporal lobe epilepsy is a continuous neurologic illness and stands as the most widespread variant of focal epilepsy with over 600,000 people in the US enduring the disorder. The majority of MTLE incidents resist anti-seizure drugs, resulting in extremely constrained treatment possibilities.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of September 7, 2023, there are 4 investigational drugs for the Kainic acid receptor target, including 5 applicable indications,5 R&D institutions involved, and as many as 50 patents.
AMT-260 is an AAV9 gene therapy treatment, using miRNA silencing technology, developed to minimize the expression of the GRIK2 gene responsible for GluK2 containing kainate receptors. By reducing the disproportionate number of these receptors which are thought to incite epilepsy particularly when their presence is altered in the epileptic hippocampus, the therapeutic aim is achieved. AMT-260 represents a novel potential one-time administered approach to treating refractory MTLE.