Unveiling the Secrets of IL-23 Inhibitors: Stay Updated with the Latest Advances
IL-23, or Interleukin-23, is a crucial cytokine that plays a significant role in the human body's immune response. It is primarily produced by activated antigen-presenting cells, such as dendritic cells and macrophages. IL-23 acts as a mediator between innate and adaptive immunity by stimulating the production of pro-inflammatory cytokines and promoting the differentiation and expansion of T-helper 17 (Th17) cells. Th17 cells, in turn, produce various cytokines that contribute to inflammation and immune responses against pathogens. However, dysregulation of IL-23 signaling has been associated with several autoimmune diseases, making it an important target for therapeutic interventions in the pharmaceutical industry.
Interleukin-23 (IL-23) inhibitors are a class of therapeutics that target the cytokine IL-23, which plays a crucial role in inflammation and autoimmune diseases. IL-23 was first discovered 20 years ago in an in silico bioinformatics search for novel members of the IL-6 cytokine family. The first agents targeting IL-23 to be investigated in humans were briakinumab and ustekinumab.
IL-23 inhibitors are expected to continue to play a significant role in the treatment of various diseases. They have shown promising results in treating serious cases of psoriasis and are also used widely for the treatment of rheumatoid arthritis. However, the emergence of resistance and the lack of an effective reversal drug when bleeding occurs are challenges that need to be addressed. Ongoing research and development efforts are focused on addressing these issues and expanding the clinical applications of IL-23 inhibitors. The approval of these drugs in more countries would further contribute to addressing the unmet medical needs of individuals with immune system diseases, digestive system disorders, and skin and musculoskeletal diseases.
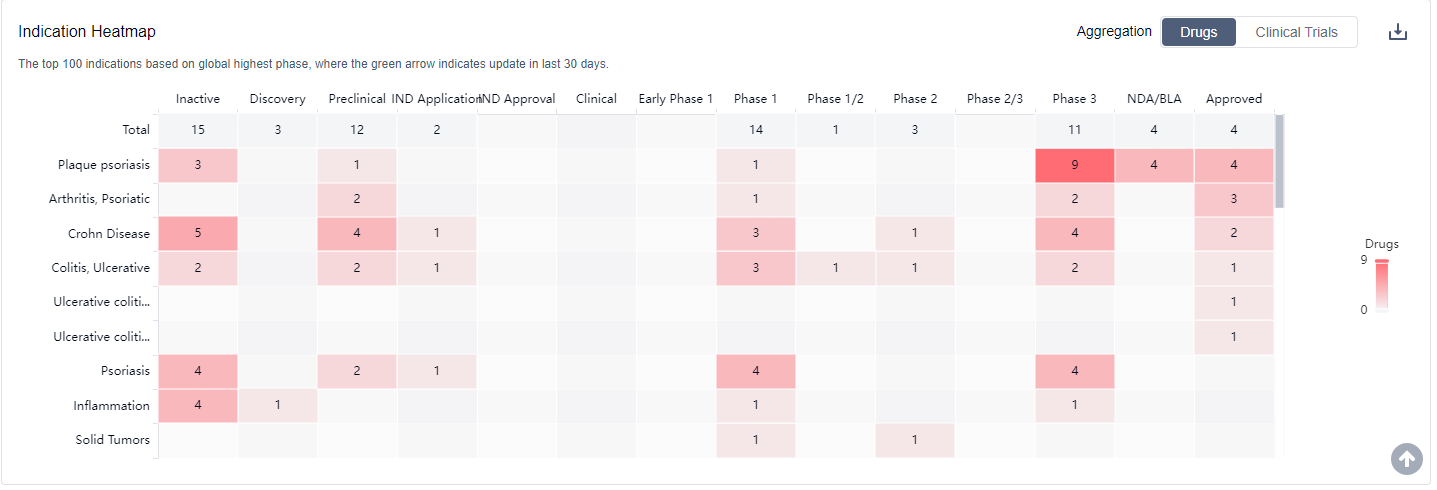
The analysis of target IL-23 in the pharmaceutical industry reveals a competitive landscape with multiple companies actively developing drugs in various stages of development. Alvotech Swiss AG, Johnson & Johnson, Amgen, Inc., AstraZeneca PLC, Celltrion, Inc., and Biocon Ltd. are among the companies growing fastest under this target. Drugs targeting IL-23 have been approved for indications such as plaque psoriasis, arthritis psoriatic, and Crohn's disease. Monoclonal antibodies, biosimilars, and small molecule drugs are progressing most rapidly under this target, indicating intense competition. Japan, the United States, and China are the countries/locations developing fastest under this target, with China showing significant progress. Overall, the future development of target IL-23 in the pharmaceutical industry is promising, with a focus on innovative drugs and expanding indications.
How do they work?
IL-23 inhibitors are a type of medication that target and inhibit the activity of interleukin-23 (IL-23), which is a cytokine involved in the immune response. From a biomedical perspective, IL-23 inhibitors are used in the treatment of various autoimmune diseases, particularly those involving chronic inflammation, such as psoriasis and psoriatic arthritis. IL-23 plays a crucial role in the development and maintenance of these conditions by promoting the activation and proliferation of immune cells, leading to excessive inflammation.
By inhibiting IL-23, these inhibitors help regulate the immune response and reduce inflammation. They can be administered through different routes, such as subcutaneous injections or intravenous infusions, depending on the specific medication. IL-23 inhibitors have shown efficacy in improving symptoms and reducing disease progression in patients with autoimmune diseases associated with IL-23 dysregulation.
It is important to note that IL-23 inhibitors specifically target IL-23 and do not affect other cytokines or immune pathways. This targeted approach helps minimize potential side effects and provides a more tailored treatment option for patients with IL-23-driven autoimmune conditions. IL-23 inhibitors represent a significant advancement in biomedicine, offering new therapeutic options for managing chronic inflammatory diseases.
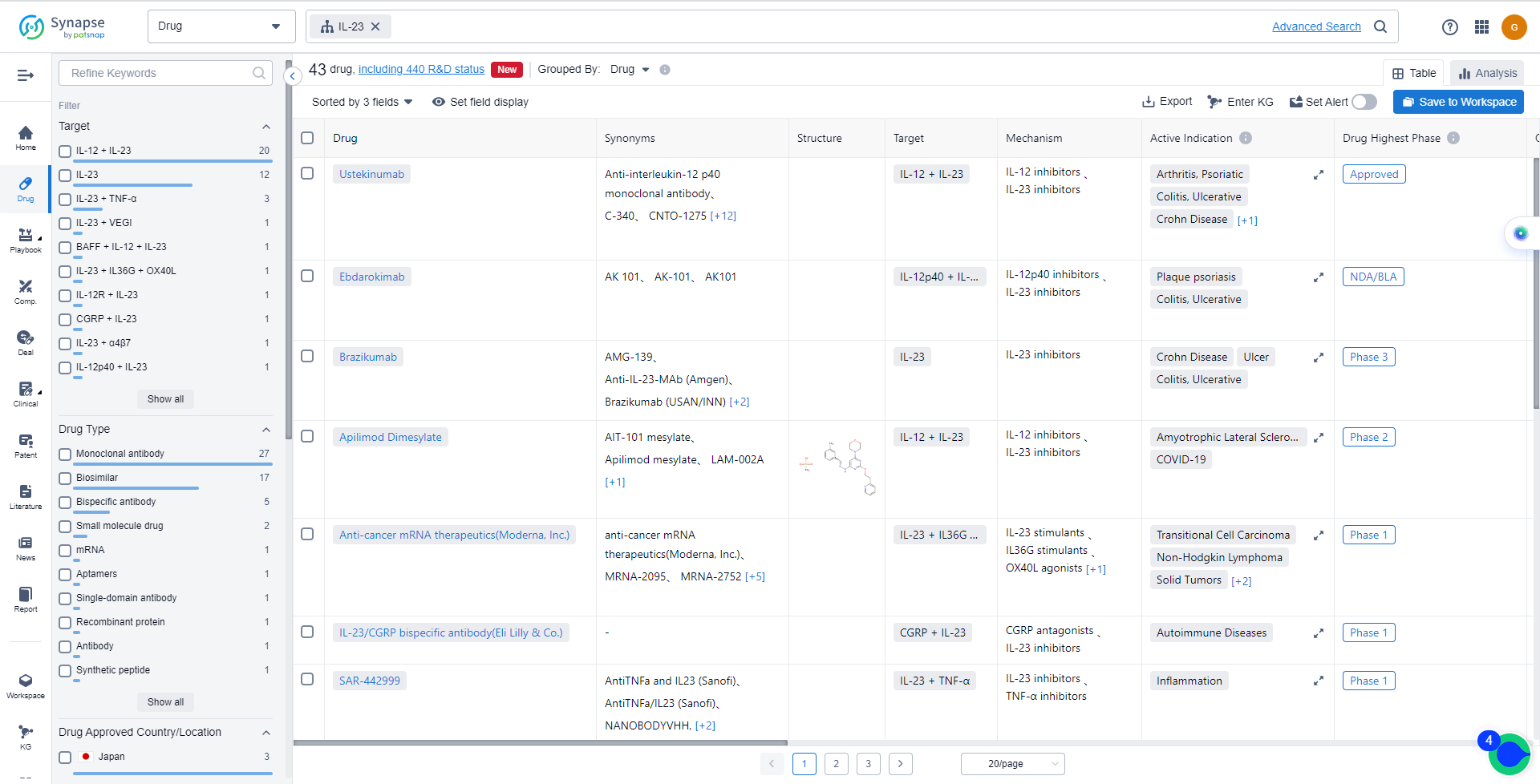
List of IL-23 Inhibitors
The currently marketed IL-23 inhibitors include:
- Ustekinumab
- Ebdarokimab
- Brazikumab
- Apilimod Dimesylate
- Anti-cancer mRNA therapeutics(Moderna, Inc.)
- IL-23/CGRP bispecific antibody(Eli Lilly & Co.)
- SAR-442999
- SFA-002
- SOR-102
- CMX-02
For more information, please click on the image below.
What are IL-23 inhibitors used for?
IL-23 inhibitors have shown promising results in treating serious cases of psoriasis and are also used widely for the treatment of rheumatoid arthritis. For more information, please click on the image below to log in and search.
How to obtain the latest development progress of IL-23 inhibitors?
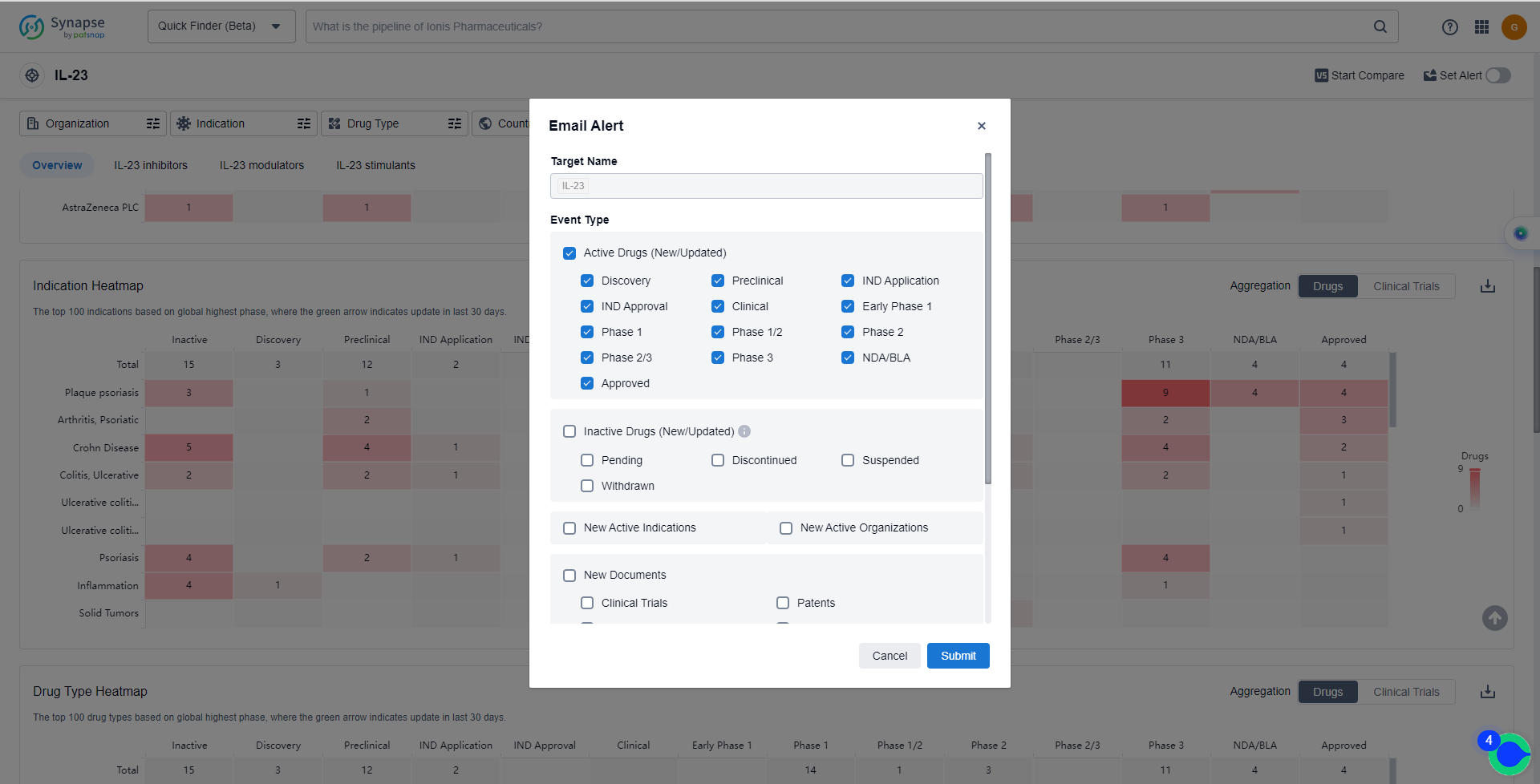
In the Synapse database, you can keep abreast of the latest research and development advances of IL-23 inhibitors anywhere and anytime, daily or weekly, through the "Set Alert" function. Click on the image below to embark on a brand new journey of drug discovery!