Utilizing Drug Names to Uncover Related Biological Sequences for Drug Development
The Center for Drug Evaluation, NMPA (CDE) shows that the application for the listing of Overland Pharmaceutical's CD19 antibody-drug conjugate (ADC), Loncastuximab, has been accepted. This is also the first China-reported ADC targeting CD19. CD19 is a popular target in the development of antitumor drugs. If Loncastuximab is approved, multiple types of drugs, including monoclonal antibodies, bispecific antibodies, CAR-T cells, and ADCs, will fulfill this target. We take Loncastuximab as an example to retrieve its related biological sequences.
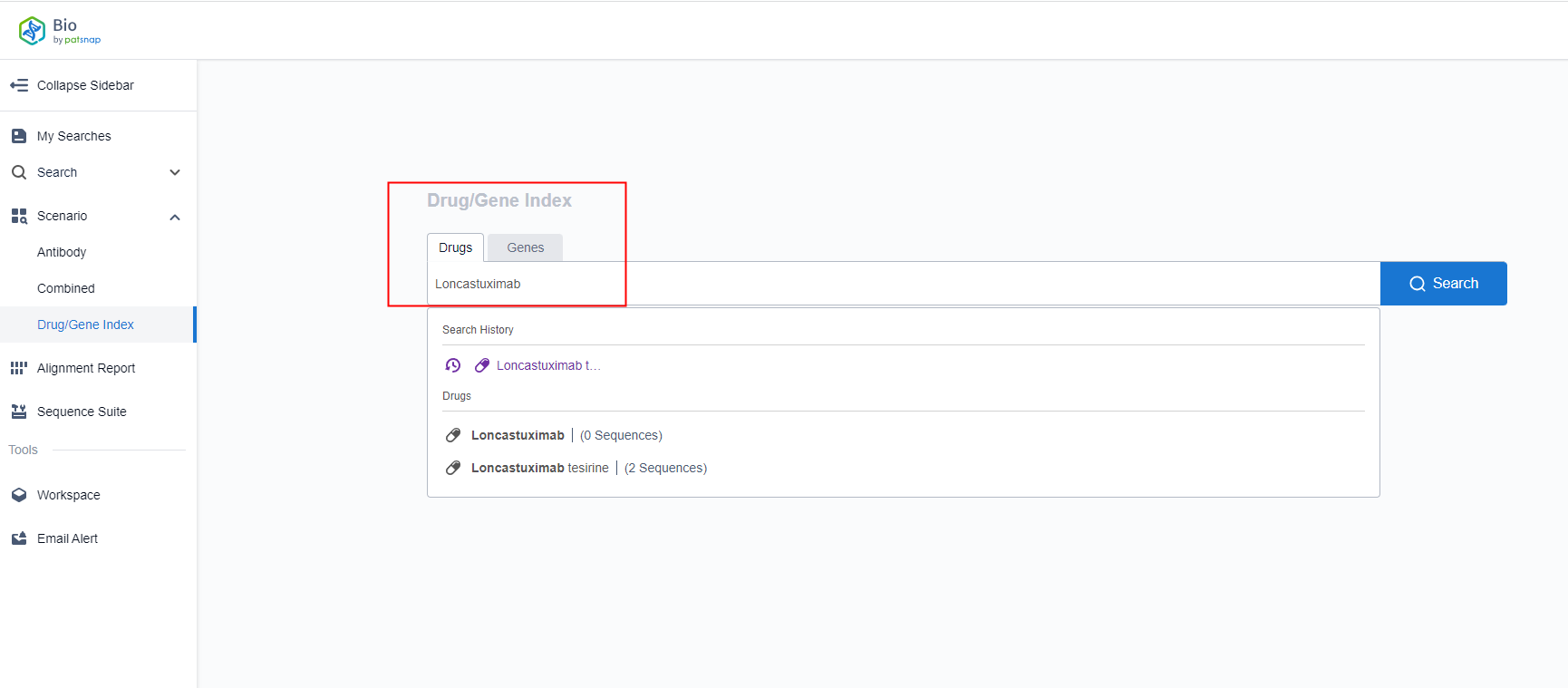
Firstly, you can apply for a free account in the Patsnap Bio Sequence Database. On the homepage of the database, in the 'Drug/Gene Index' of the scene search on the left, you can enter 'Loncastuximab' or its commercial name to conjure up entities and show the corresponding sequence quantity. Click to search and enter the detail page.
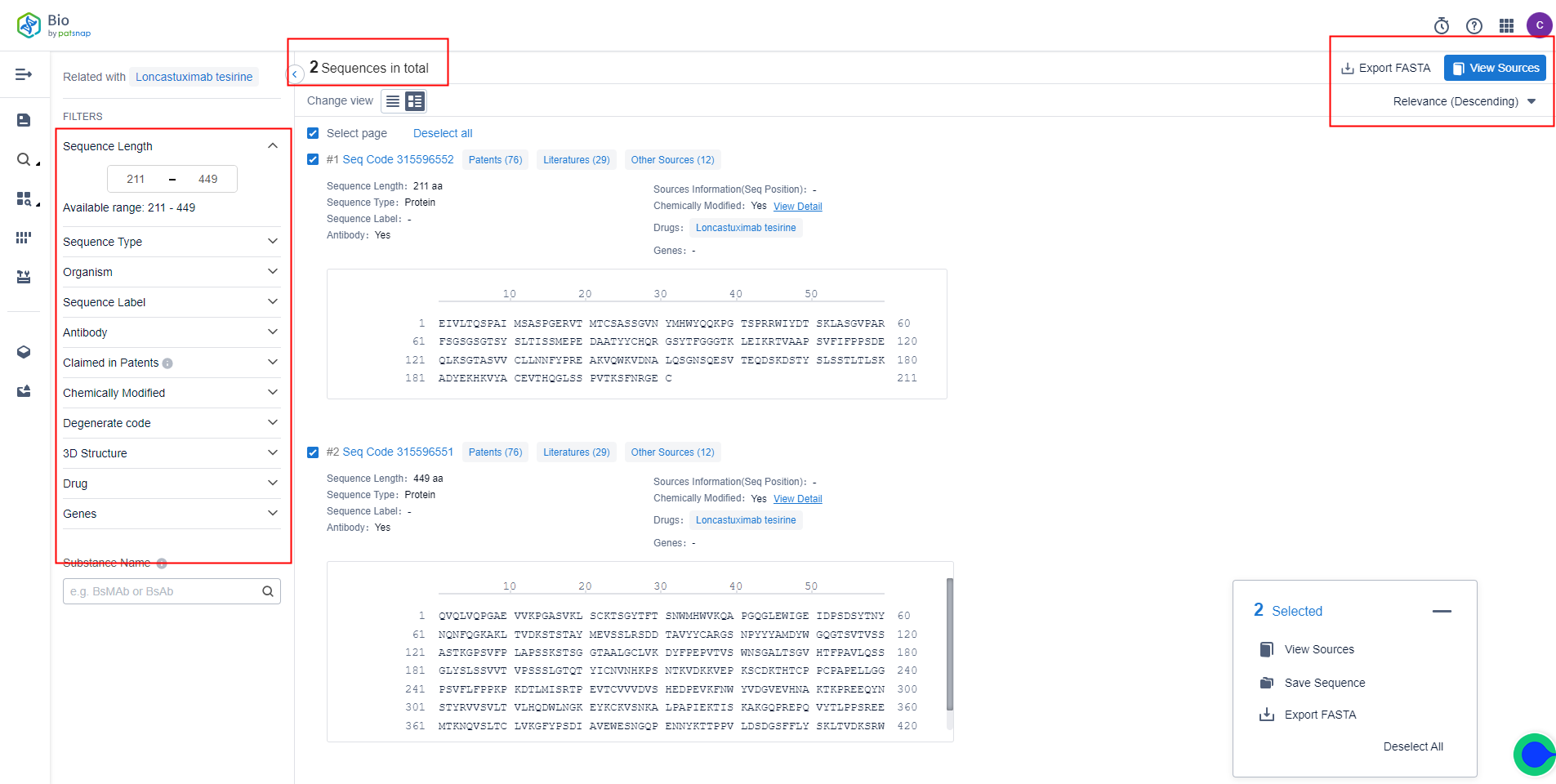
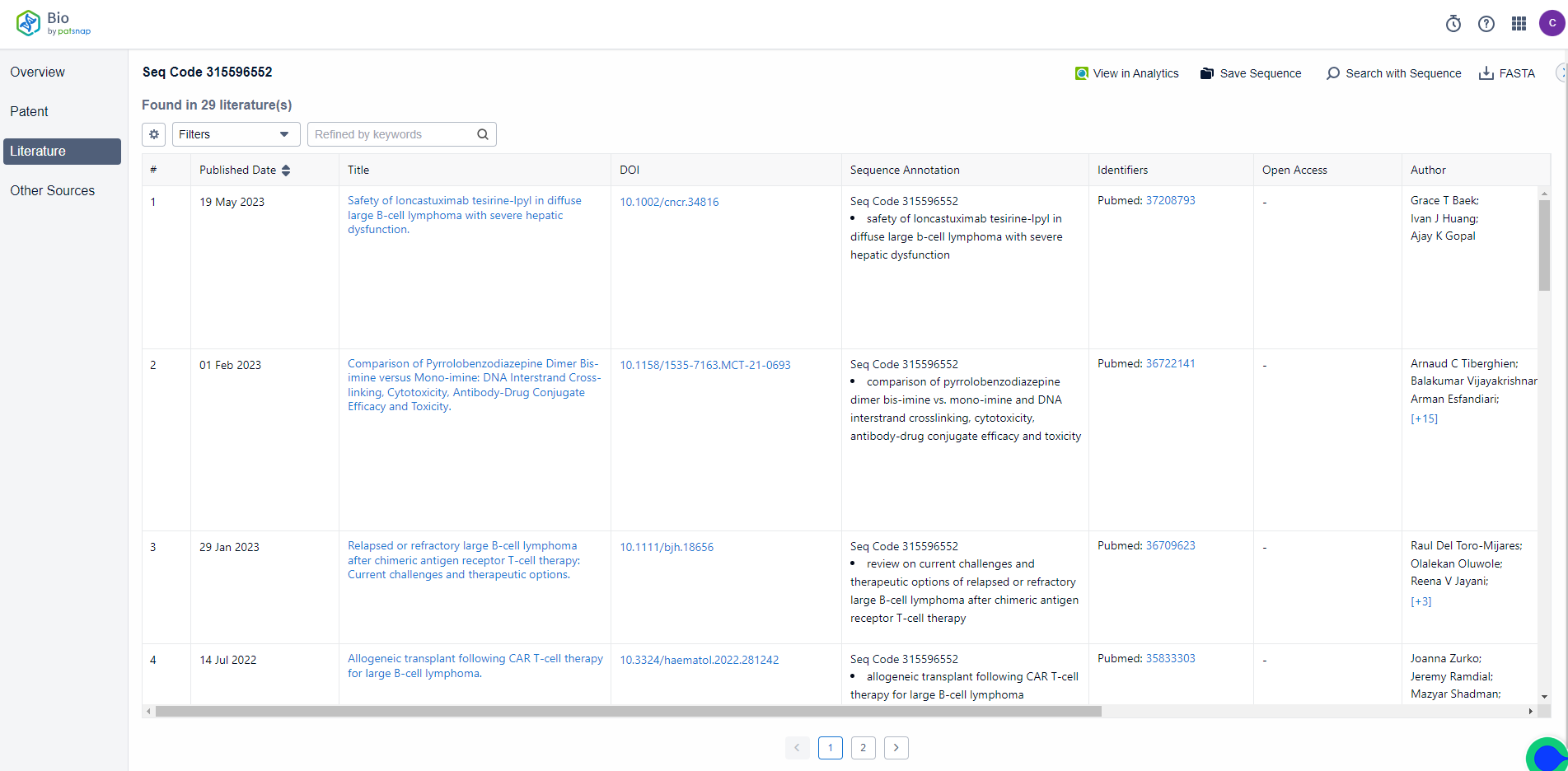
The detail page shows two sequence results of Loncastuximab. You can also customize sequence length, sequence type, organism, and whether there are modified sequences in the filter on the left. Selected sequences can be exported to the local drive with one click as a FASTA file. There are 76 patents, 29 literature materials, and other sources of data related to the sequence of Loncastuximab on the results page. Click to see the public source for data details.
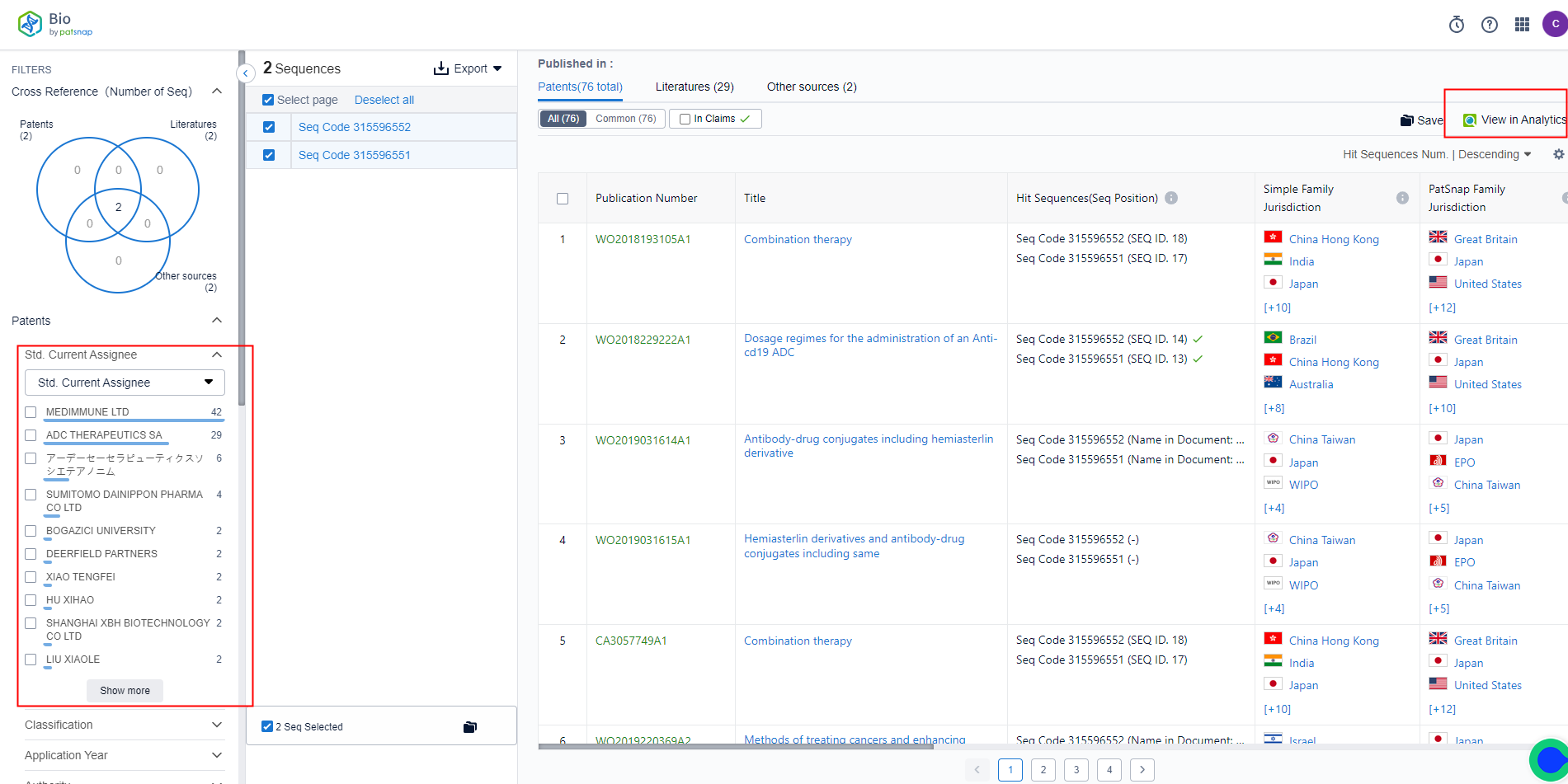
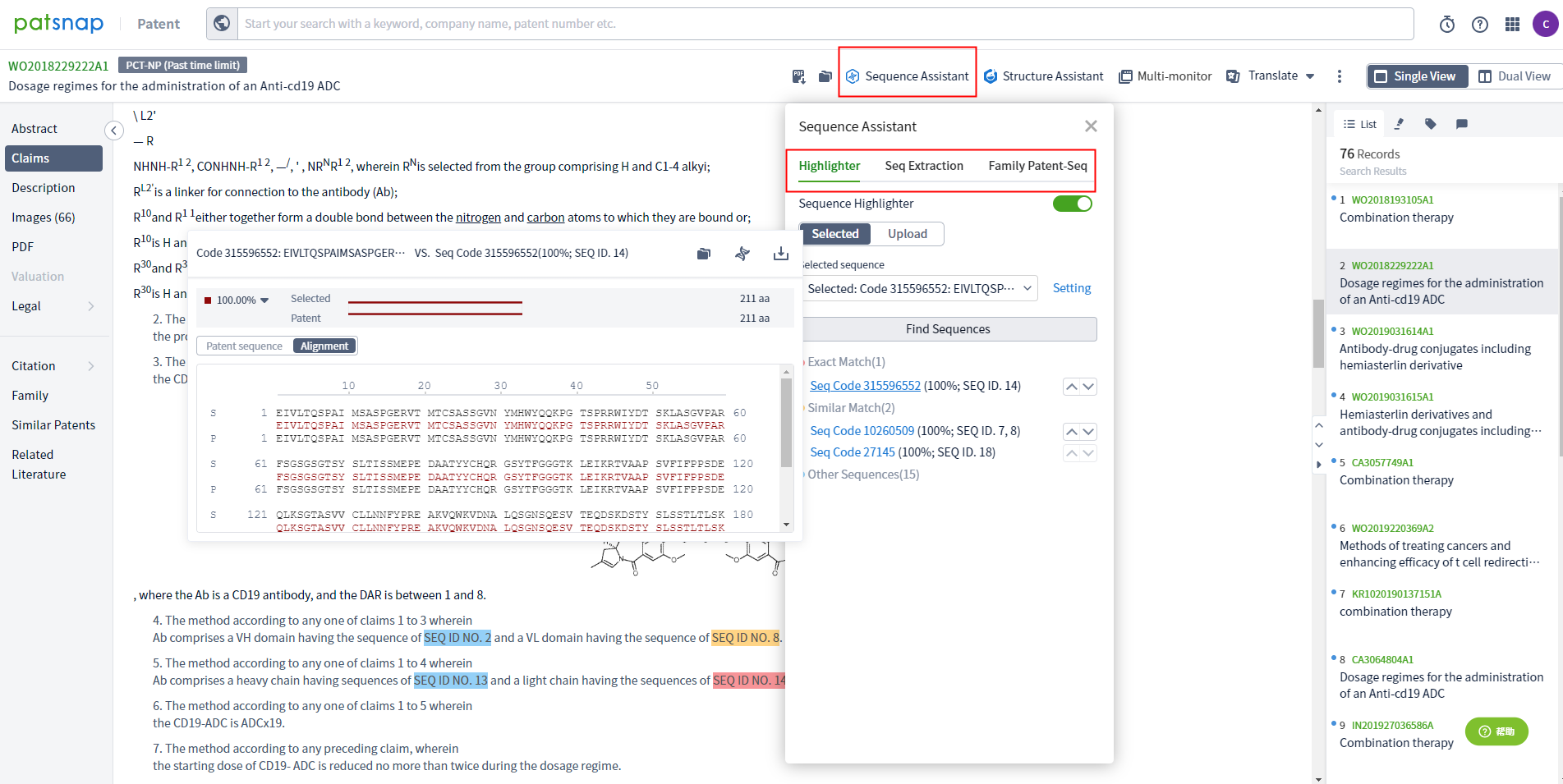
By clicking on patents, you can directly jump to the patent database to view the details of the patent. You can also use the filter on the left for precise screening. The sequence assistant on the patent detail page can be used to highlight the selected sequence for matching.
The Literature database data show the publication date, title, and DOI link of related literature, which allows direct access to the original text. It also includes sequence annotation and Pubmed identifiers.
It is important to note that Patsnap Bio is the most extensive sequence search platform for the Patsnap database. It incorporates AI with human-curated data for comprehensive handling of protein and nucleotide sequence data plucked from global patents, biological periodicals, and public repositories. Essential biological sequences are manually annotated illuminating structural modifications to provide the most accurate sequence data and boost sequence retrieval efficiency.
Free registration is currently available to utilize the Bio biological sequence database: https://bio-patsnap-com.libproxy1.nus.edu.sg. Act now to expedite your sequence search tasks.