VBI vaccine release brii-179 (vbi-2601) combined with PEG-IFN α Results of the second phase study on the treatment of chronic hepatitis B
VBI Vaccines Inc. publicized that its strategic HBV collaborator, Brii Biosciences announced preliminary group-level unmasked 36-week data from a mid-term examination of a randomly assigned, placebo-controlled, and double-concealed Phase 2 investigation of BRII-179 (VBI-2601). This innovative Pre-S1/Pre-S2/S therapeutic vaccine, when combined with PEG-IFNα, was tested on chronic hepatitis B virus patients and compared with the PEG-IFNα only treatment. In earlier studies, it was observed by both VBI and Brii Bio that BRII-179 (VBI-2601) evoked extensive antibody and T-cell responses against Pre-S1, Pre-S2, and S epitopes in HBV patients.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"We applaud Brii Bio's enthusiasm towards this data and their ongoing swift progress in executing clinical programs, as they persist in their quest to elevate the functional cure rate for hepatitis B," affirmed Jeff Baxter, CEO and President of VBI. "Brii Bio's unwavering devotion, investments, and collaborations in this area continue to underscore the strategic value of our alliance. We eagerly anticipate additional data from this investigation and continued studies of this premier immunotherapeutic contender."
In the month of July 2023, both VBI and Brii Bio publicized an extension to their HBV collaboration involving exclusive international rights to develop and market BRII-179 (VBI-2601), alongside an exclusive right to mature and sell PreHevbri®, VBI's 3-antigen mature HBV preventive vaccine, excluding Japan, within the Asia Pacific district.
The Phase 2 trial, a placebo-controlled, double-blind, and randomized research conducted across various centers, strives to analyze the safety and efficacy of BRII-179 (VBI-2601) when used as a supplemental therapy alongside PEG-IFNα and NrtI treatment for chronic HBV infection. The said trial included adult HBV patients in mainland China who according to treatment guidelines had received 24 to 28 doses of PEG-IFNα and minimum 12 months of NrtI therapy, also meeting a predetermined criteria for partial response.
Participants were allocated at 1:1 to either receive BRII-179 (VBI-2601) or placebo every three weeks. This involves a total of 7 doses spread across 18 weeks, while continuing PEG-IFNα treatment for a period of 48 weeks. Subjects meeting the Nrtl discontinuation criteria would discontinue Nrtl treatment followed by an additional 48 weeks of follow-up.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
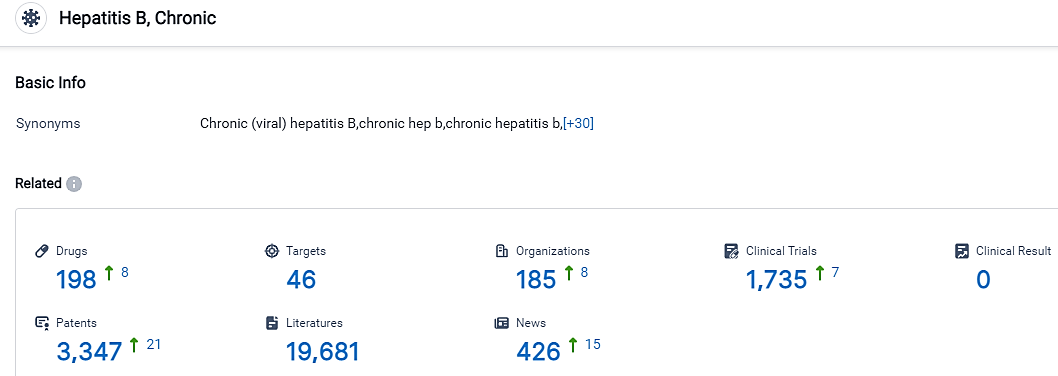
According to the data provided by the Synapse Database, As of September 8, 2023, there are 198 investigational drugs for the Chronic Hepatitis B, including 46 targets,185 R&D institutions involved, with related clinical trials reaching 1735,and as many as 3347 patents.
A unique recombinant, protein-based HBV immunotherapeutic candidate known as BRII-179 (VBI-2601) has been developed to stimulate enhanced and vast B-cell and T-cell immunity. This proposition was licensed globally to Brii Biosciences under an exclusive development and licensing contract. At present, BRII-179 (VBI-2601) is undergoing testing in a pair of Phase 2 clinical trials, where it is combined with BRII-835 (VIR-2218) and PEG-IFNα. This combination is part of a potential therapeutic regime aimed at functionally curing persistent HBV infection.