Viatris: Layout of Potential Products for Acute Myocardial Infarction and Systemic Lupus Erythematosus
On February 28th, Viatris, a pharmaceutical company based in the United States, and Idorsia, a Swiss pharmaceutical company, announced the establishment of a significant global research and development collaboration. Under the terms of the agreement, Viatris will gain global exclusive rights to develop and commercialize two Phase 3 clinical-stage drugs (Selatogrel and Cenerimod) from Idorsia and may potentially expand cooperation to include additional innovative assets in the future.
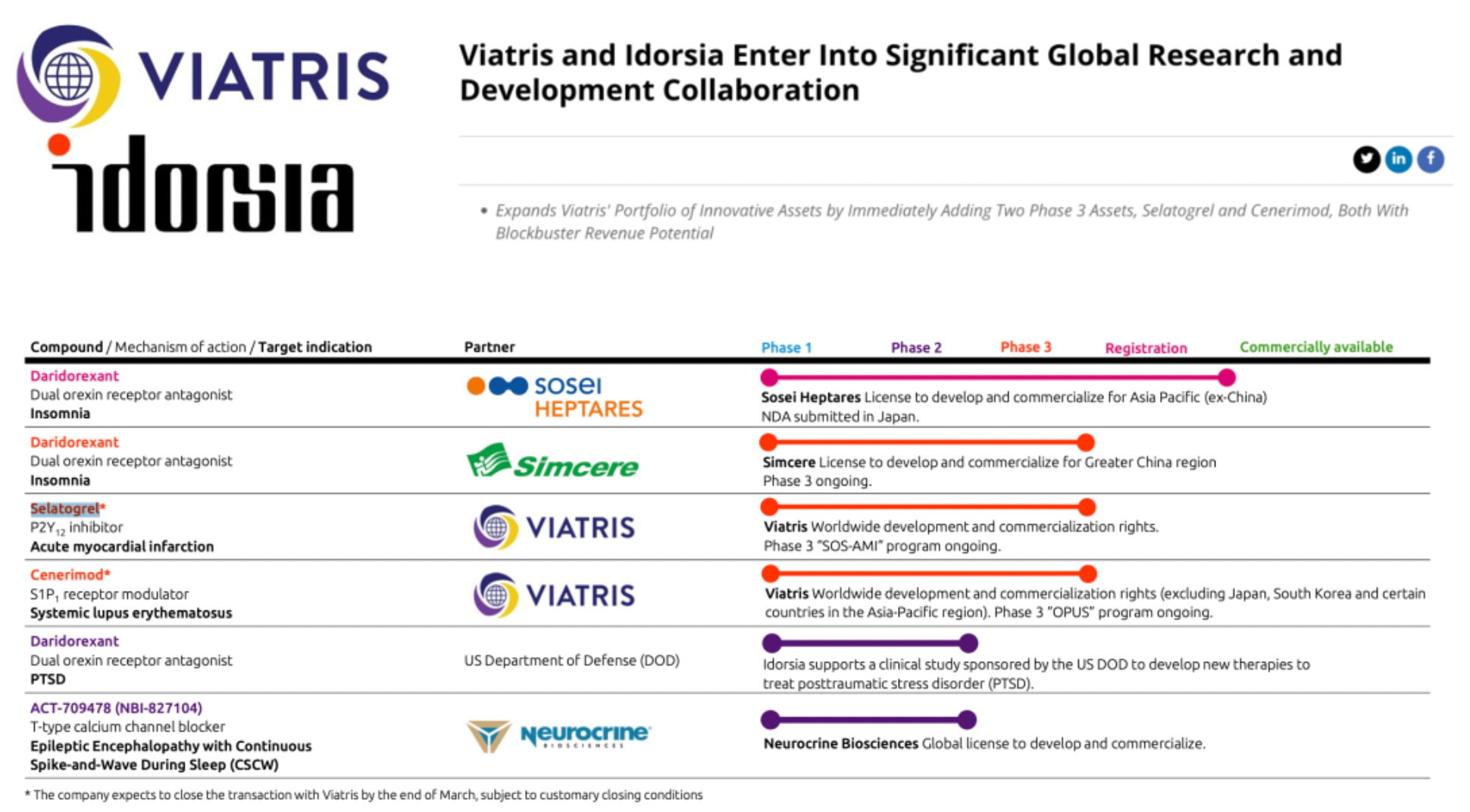
Selatogrel is reported to be a potential life-saving medication for patients with a history of acute myocardial infarction (AMI), designed to be administered by the patients themselves. This drug is a fast-acting, reversible, and highly selective P2Y12 inhibitor, intended for early treatment during the onset of AMI to protect cardiac muscle tissue. It is aimed at preventing worsening of heart conditions and reducing mortality risk during the critical timeframe from the emergence of symptoms to the first medical intervention.
According to Idorsia, a large-scale international multicenter, double-blind, randomized controlled Phase 3 study named "Study of Selatogrel in Suspected Acute Myocardial Infarction" (SOS-AMI) is being conducted to evaluate the clinical efficacy and safety of self-administered Selatogrel at 16mg on top of standard care at the time of suspected AMI symptoms. This study has been granted a Special Protocol Assessment agreement by the U.S. FDA and has received "Fast Track" designation, indicating its role in fostering communication and collaboration for the development of drugs for serious conditions with unmet medical needs.
Cenerimod, as a result of two decades of research in Idorsia's laboratories, has emerged as a highly selective S1P1 receptor modulator, available in a once-daily oral tablet form. This drug could offer a new therapeutic approach for systemic lupus erythematosus (SLE), a serious disease that significantly impacts patients' lives and for which current treatment options are limited.
In December 2022, Idorsia launched a project named OPUS (Oral S1P1 Receptor Modulation for SLE), which included two multicenter, randomized, double-blind, placebo-controlled phase III studies. The aim of the study is to evaluate the efficacy, safety, and tolerability of cenerimod 4mg in adults with moderate to severe SLE on top of standard therapy. The primary endpoint is to demonstrate the improvement in disease activity and control of the condition with cenerimod compared to placebo at 12 months, with the primary endpoint being the achievement of an SRI-4 response from baseline. Similarly, cenerimod's use in the treatment of SLE has also been granted "Fast Track" designation by the FDA.
Through this collaboration, Viatris leverages its strong financial position and global operational infrastructure, combined with Idorsia's mature and efficient drug development team and their innovative capabilities, to jointly advance the development and commercialization of these two potential blockbuster drugs as well as other future innovative assets.
Under the terms of the agreement, Idorsia will transfer the development projects of Selatogrel and Cenerimod, along with some related personnel, to Viatris. In exchange, Viatris will make an upfront payment of $350 million to Idorsia, with potential additional milestone payments dependent on development and regulatory approval progress. In addition, once these two drugs achieve annual net sales, Viatris will pay Idorsia tiered sales milestone payments as well as royalties at a percentage ranging from high single-digits to low double-digits. Both Viatris and Idorsia will jointly bear the costs of developing these two drugs.
To oversee the further development of these two phase III clinical stage projects until regulatory approval, the parties will establish a joint development committee to provide guidance. Furthermore, the agreement also grants Viatris the right of first refusal and first negotiation for other specific assets in Idorsia's pipeline, allowing Viatris the right to firstly decline or accept proposals for collaboration on these assets under certain conditions.
Acute Myocardial Infarction
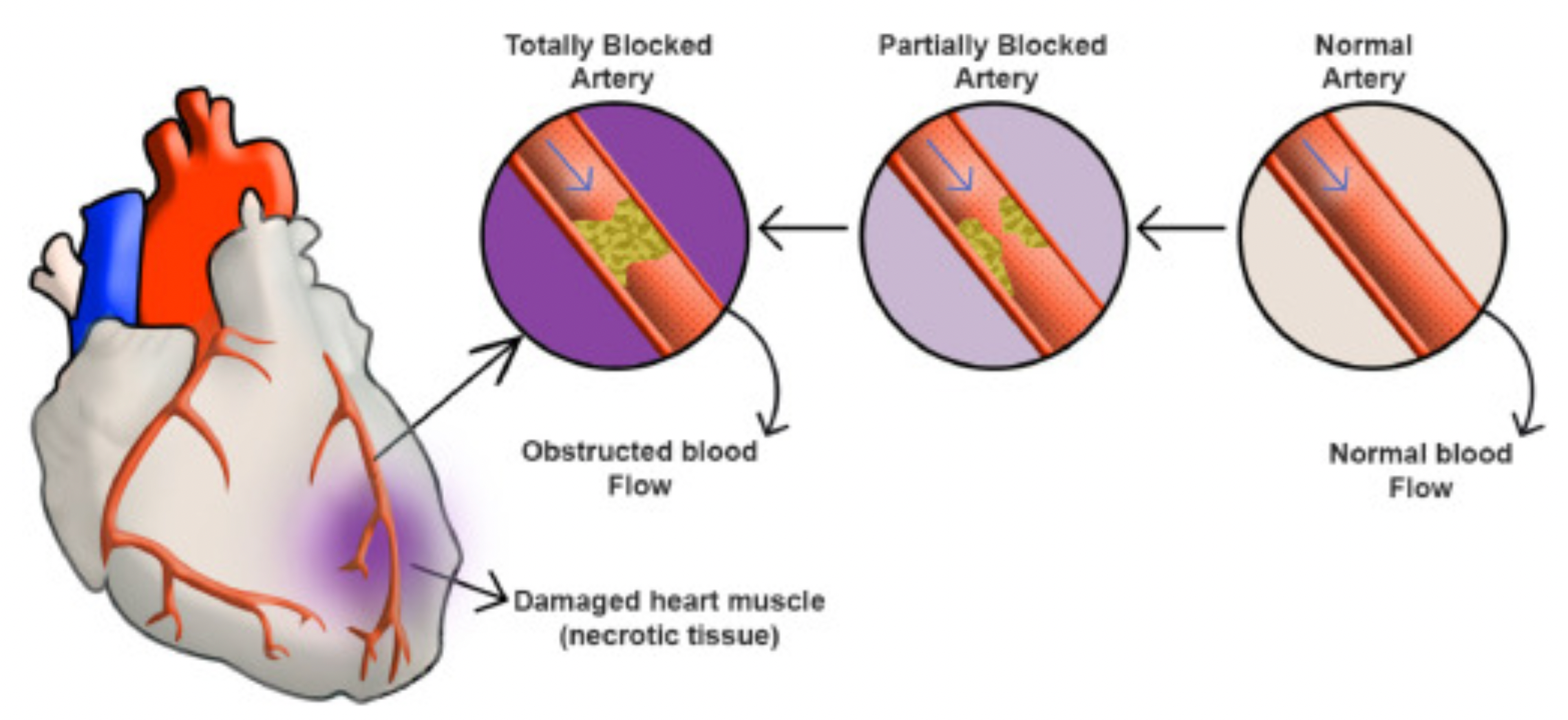
Acute Myocardial Infarction (AMI) refers to the pathological process in which myocardial cells undergo necrosis due to sudden obstruction of the coronary arteries leading to an interruption of blood flow. This condition is primarily caused by the rupture of atherosclerotic plaques in the coronary arteries, secondary thrombosis formation, or other reasons leading to acute occlusion of the coronary vessels.
Clinically, the main symptoms of acute myocardial infarction include, but are not limited to, chest pain (often described as crushing, constrictive or heaviness), dyspnea (which may or may not be present), nausea, and diaphoresis. Chest pain is typically persistent and may radiate to the left shoulder, left arm, neck, lower jaw, or even the back.
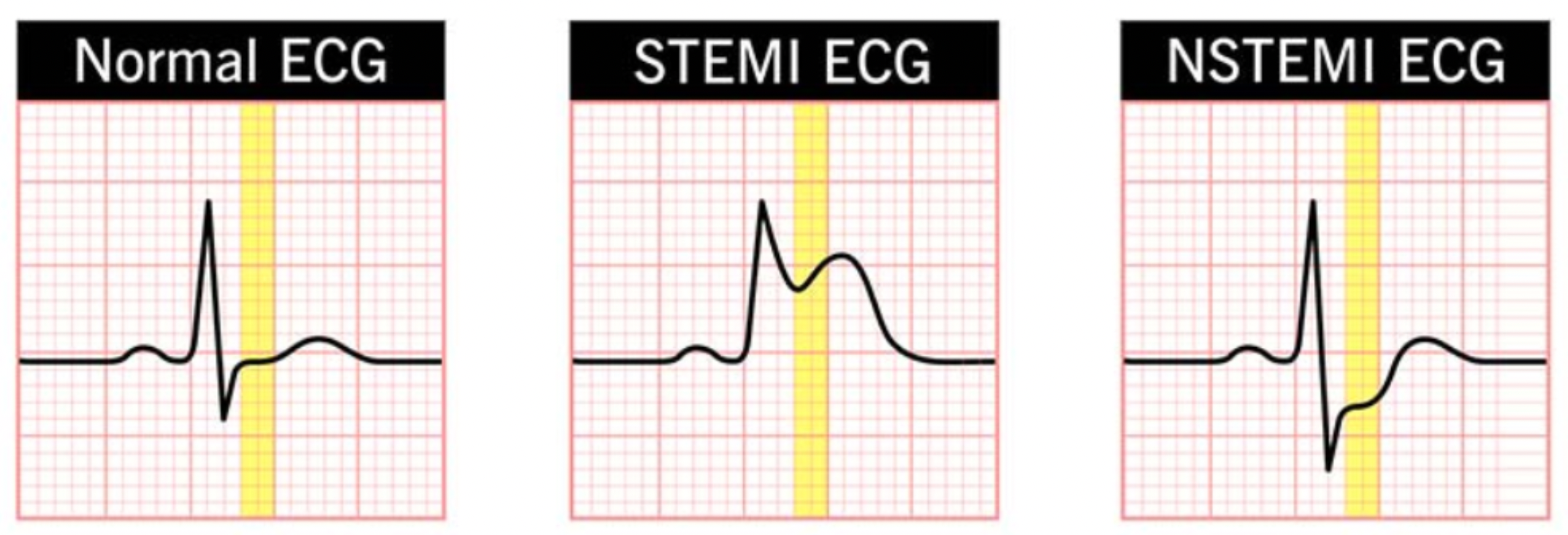
Acute myocardial infarction is divided into ST-segment elevation myocardial infarction (STEMI) and non-ST-segment elevation myocardial infarction (NSTEMI). In the case of STEMI, due to the more urgent condition and involvement of a complete occlusive coronary thrombus, urgent reperfusion therapy is required to restore myocardial blood flow. For NSTEMI, medication therapy is needed to stabilize the condition, and reperfusion treatment options are selected based on the patient's specific circumstances. When diagnosing acute myocardial infarction, doctors primarily rely on electrocardiograms and biomarkers.
The treatment for acute myocardial infarction primarily includes antiplatelet therapy, anticoagulation, nitrate medications, beta-blockers, statins, and reperfusion strategies, among others.
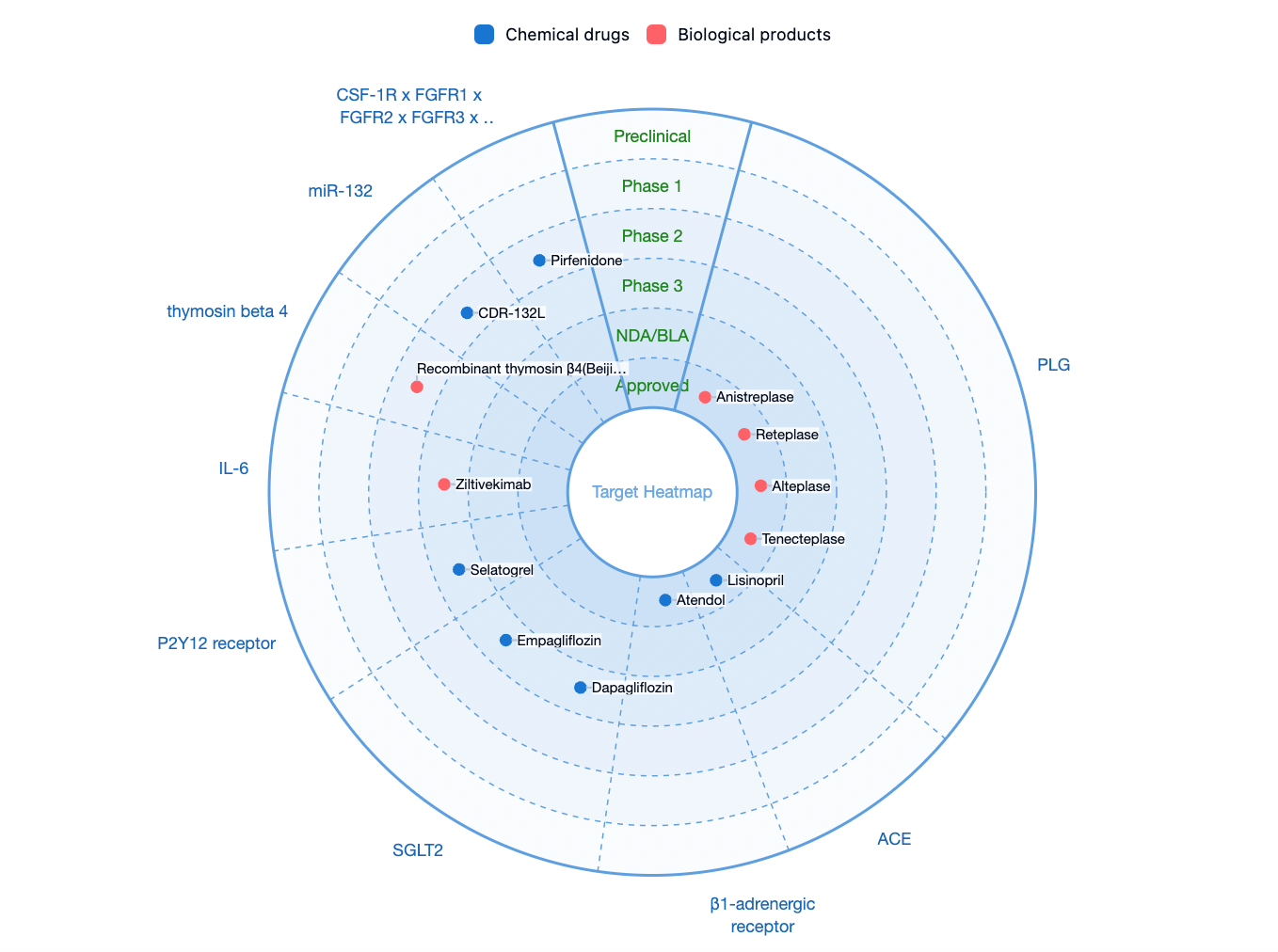
According to statistics from the Synapse database, the number of drugs currently available or in clinical phases 1/2/3 for the treatment of acute myocardial infarction is relatively limited on a global scale. The main therapeutic targets under investigation include plasminogen (PLG), angiotensin-converting enzyme (ACE), adrenergic beta-1 receptor, sodium-glucose cotransporter 2 (SGLT2), purinergic P2Y12 receptor (P2Y12R), IL-6, and others.
Selatogrel
Platelet adhesion, activation, and aggregation play a critical role in the formation of thrombosis during atherosclerosis. In this process, the interaction between adenosine diphosphate (ADP) and the P2Y12 receptor on platelets is a crucial step. Antagonizing platelet P2Y12 receptors can inhibit the processes of platelet activation and aggregation. This means that the binding of ADP to the receptor is prevented, thereby reducing platelet aggregation responses and the consequent stimulation of thrombus formation.
Selatogrel is a selective P2Y12 receptor antagonist developed by Idorsia for original research and is currently undergoing Phase 3 clinical studies aimed at patients with acute myocardial infarction.
Selatogrel was initially developed by Actelion, the predecessor of Idorsia. On January 26, 2017, Johnson & Johnson announced a full cash tender offer to acquire all outstanding shares of the Swiss pharmaceutical company Actelion at a price of $280 per share (totaling $30 billion in cash). As part of the transaction, Actelion spun off its drug research and early clinical development assets into a newly established Swiss biopharmaceutical company, Idorsia.
According to the results of a Phase 1 clinical study published in 2014, the potent, highly selective, and reversible P2Y12 receptor antagonist Selatogrel (ACT-246475) showed exposure-related platelet inhibitory effects. An exploratory formulation improved the bioavailability and antiplatelet activity of ACT-246475.

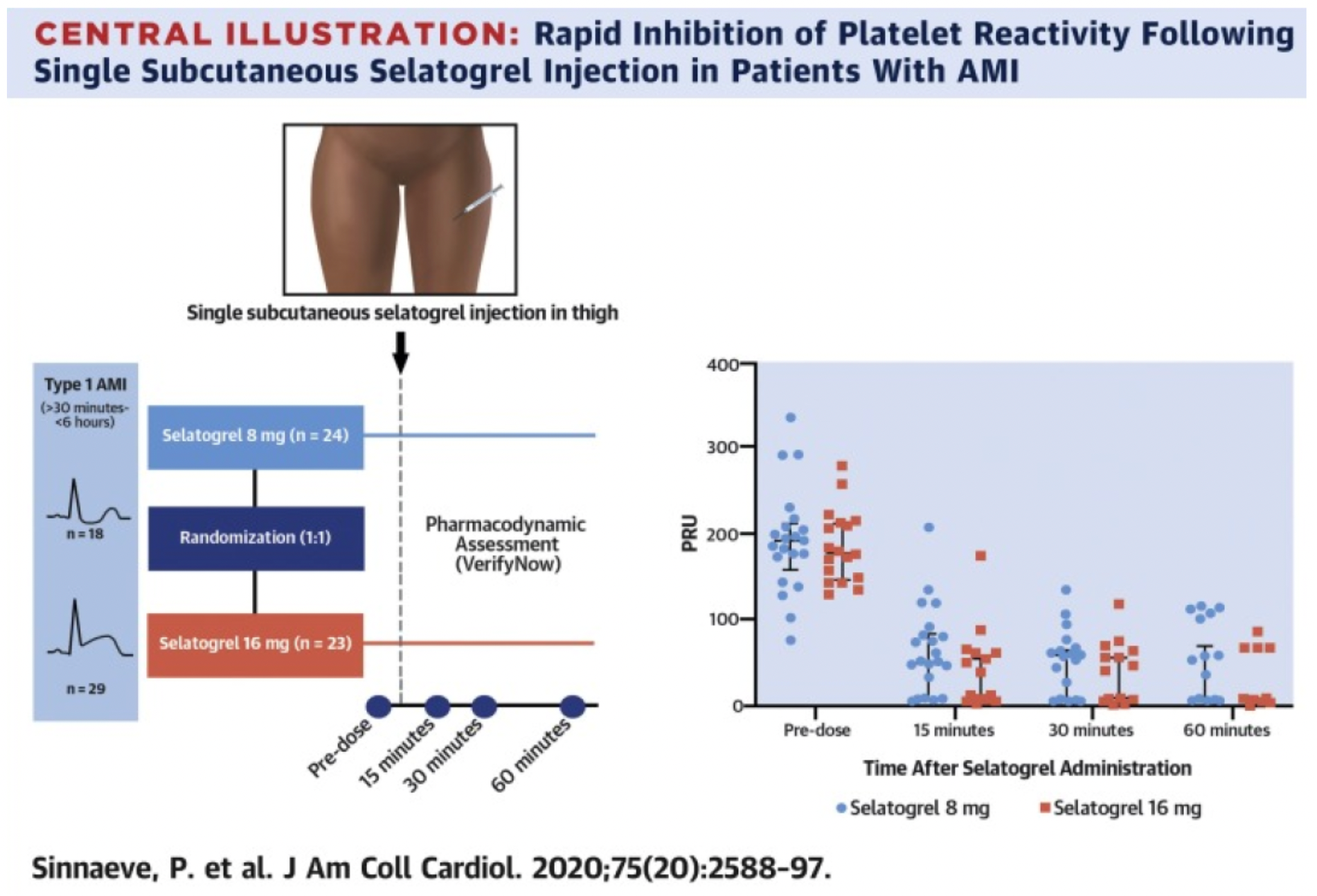
According to the results of a phase 2 clinical study published in the "J Am Coll Cardiol" journal in 2020, a total of 47 patients received treatment with Selatogrel, with 24 individuals receiving a dose of 8 mg and 23 receiving a dose of 16 mg. This was followed by 43 patients being treated with ticagrelor (another P2Y12 receptor antagonist under investigation), and one patient with clopidogrel (a P2Y12 receptor antagonist). Among patients receiving 8 mg and 16 mg doses, the proportion achieving an effective response within 30 minutes post-administration was 91% and 96%, respectively, both meeting the pre-specified efficacy target of over 85%. The response rate was not affected by the type of AMI, age, or gender. Notably, a similar response rate was observed at 15 minutes post-administration (8 mg: 75%; 16 mg: 91%), and this inhibitory effect was maintained at 60 minutes after administration (8 mg: 75%; 16 mg: 96%). The findings indicate that a single subcutaneous injection of Selatogrel is safe and feasible in AMI patients, producing significant, rapid, and dose-related antiplatelet effects.
Systemic Lupus Erythematosus
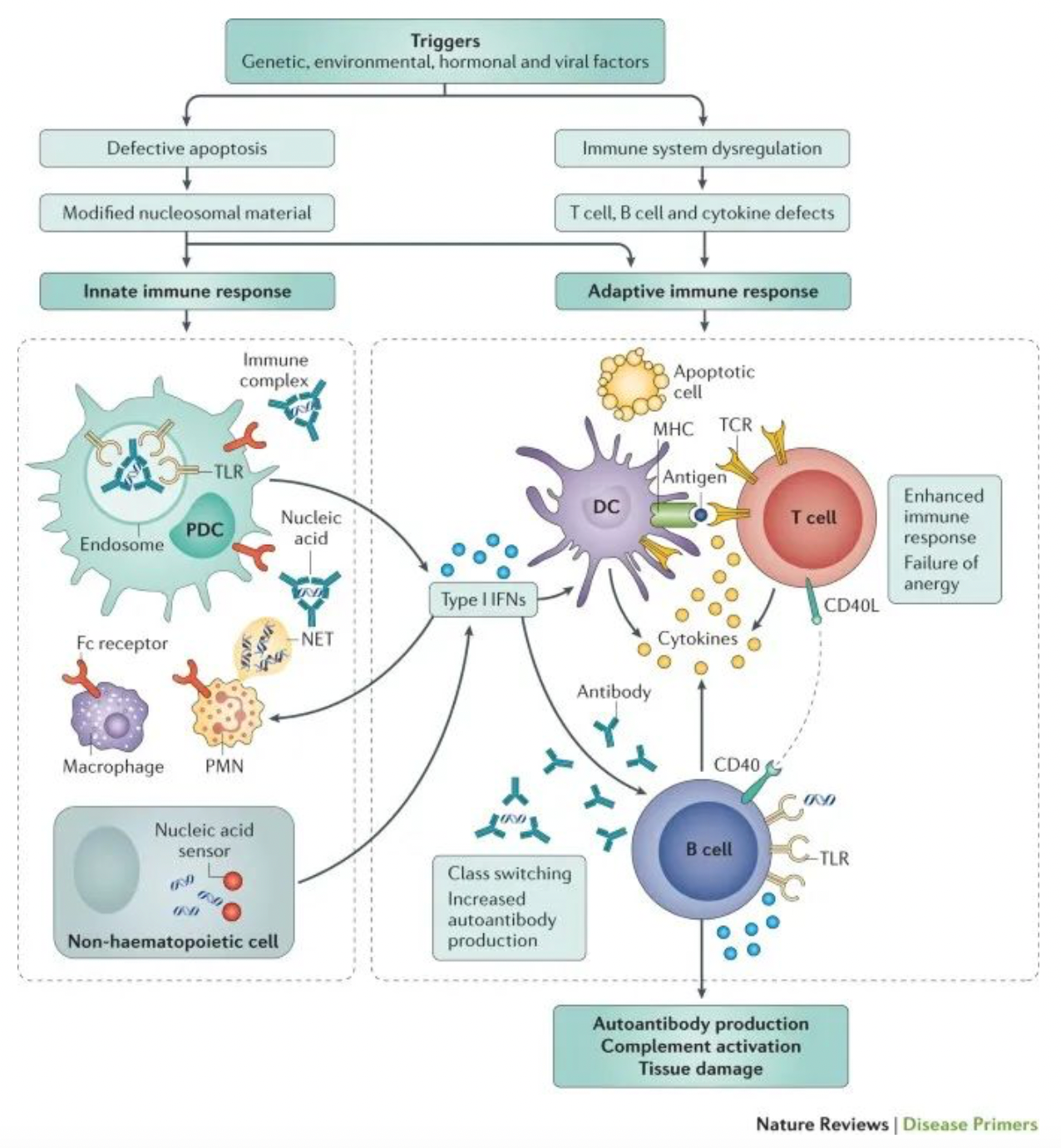
Systemic Lupus Erythematosus (SLE) is the most common type of lupus, which is an autoimmune disease. This means that the immune system becomes dysfunctional and mistakenly attacks the body's own tissues. Unlike certain autoimmune diseases that only affect a single organ, SLE has the potential to affect multiple parts of the body.
Statistics show that there are at least 5 million people worldwide living with some form of lupus, with 90% of those affected being female, most commonly developing the disease between the ages of 15 and 44. Additionally, the prevalence of lupus is higher among Asian and Afro-Caribbean populations compared to Caucasians. In SLE, the abnormal immune response can target the body's own tissues, potentially affecting the skin, joints, kidneys, heart, as well as other organs. Given the capacity of SLE to cause damage to any part of the body, the manifestations of the disease are incredibly diverse.
Therefore, the symptoms of Systemic Lupus Erythematosus (SLE) are diverse and often resemble those of many other conditions, requiring the exclusion of these similar symptoms before a definitive diagnosis can be made. This leads to SLE being frequently overlooked or misdiagnosed. However, early diagnosis is crucial for controlling lupus symptoms, initiating treatment to reduce the risk of long-term complications, and accessing a broader range of patient support services, such as local patient groups.
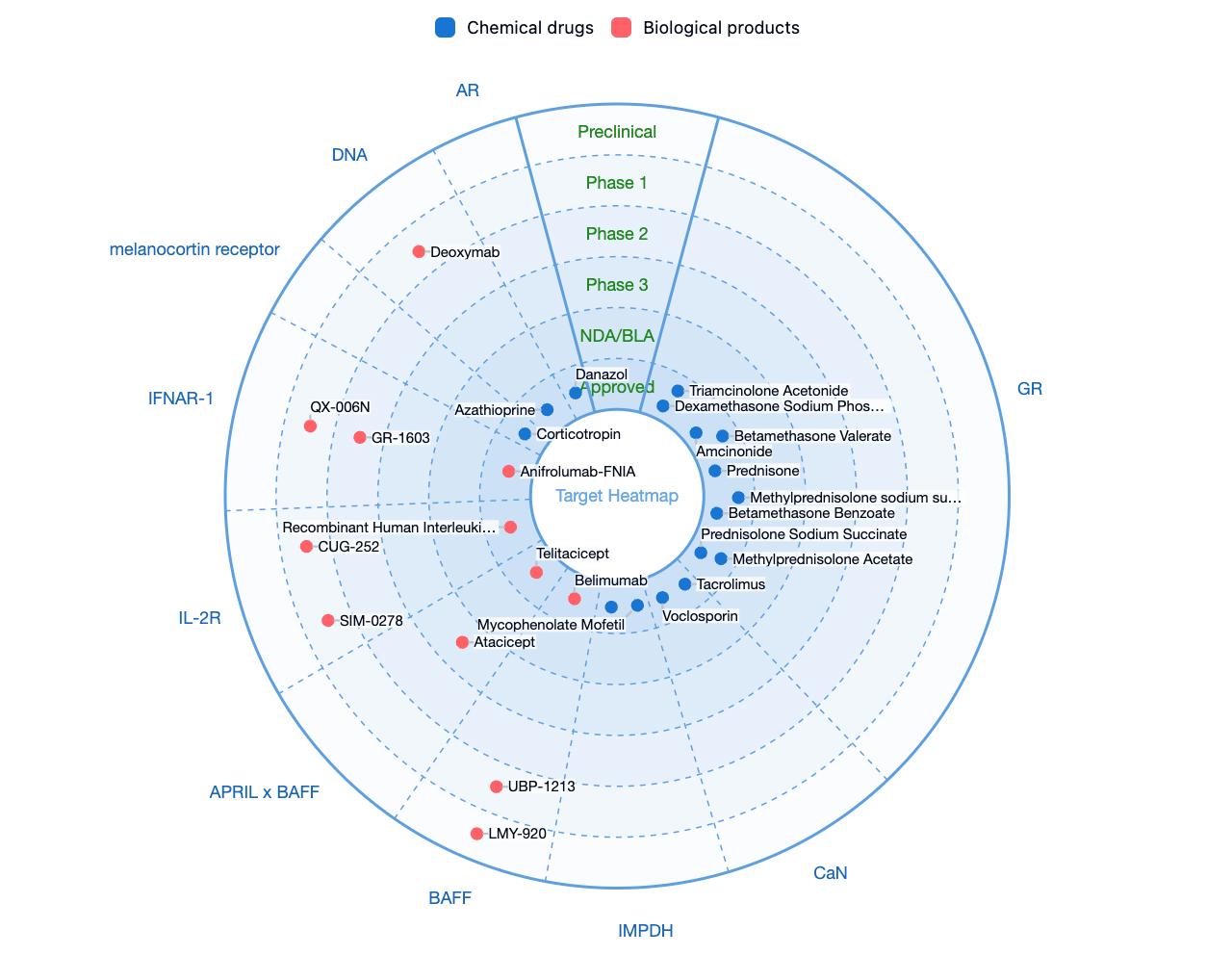
According to the Synapse database, there are currently approximately 400 therapeutic drugs and candidates in the pipeline worldwide for the treatment of SLE, whether on the market or in the research and development stage. Key therapeutic targets include glucocorticoid receptor (GR), calcineurin (CaN), inosine monophosphate dehydrogenase (IMPDH), and B-cell activating factor (BAFF), among others. Potential G-protein-coupled receptor (GPCR) family targets for the disease include melanocortin receptors, S1PR1, CXCR5, CXCR4, and others.
Cenerimod
Although the exact etiology of Systemic Lupus Erythematosus (SLE) has not been fully elucidated, T cells and B cells are considered to be the key immune cells contributing to the development of the disease. In SLE patients, both T cells and B cells are in a state of overactivity. The primary consequence of this hyperactivity is the infiltration of these immune cells into different tissues and the production of autoantibodies, which in turn leads to inflammation and organ damage. T cells and B cells have S1P1 receptors on their surface, which allow the lymphocytes to recognize the signaling molecule sphingosine-1-phosphate (S1P), a critical mediator in the regulation of lymphocyte egress from lymph nodes to the bloodstream.
Cenerimod is a selective S1PR1 modulator developed by Idorsia for clinical use in the treatment of systemic lupus erythematosus and liver injury. As an S1P1 receptor modulator, Cenerimod can inhibit the outflow of T cells and B cells from the lymph nodes. This prevents their migration and infiltration into various organs, thereby influencing the immune response that leads to the multisymptom manifestation of lupus at a fundamental level of the immune cascade.
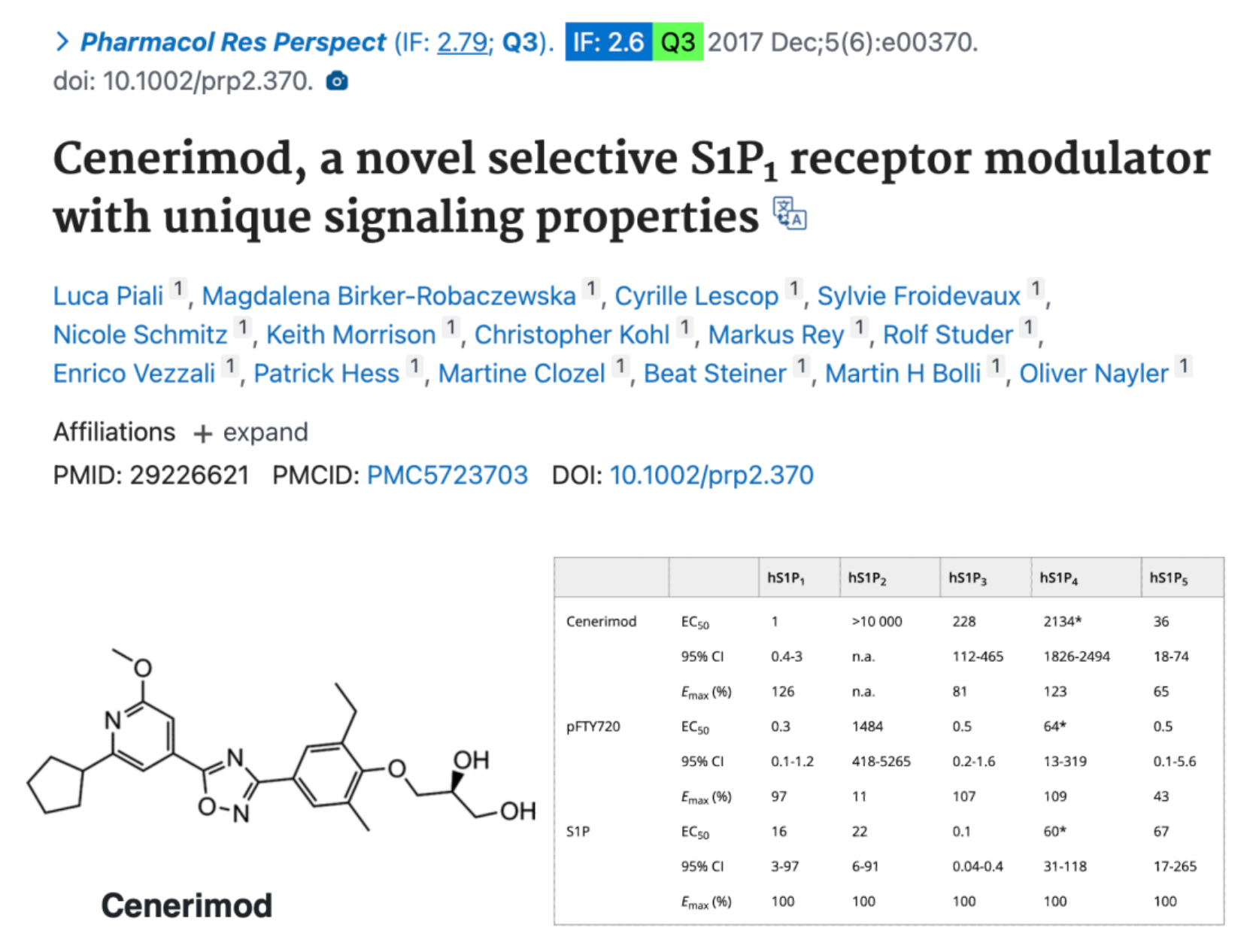
According to preclinical study data published in 2017, Cenerimod is a novel, highly efficient, and selective S1P1 receptor modulator that possesses unique S1P1 receptor signaling properties and has not observed side effects that cause bronchoconstriction or vasoconstriction in in vitro and in vivo experiments. Administered orally, Cenerimod can dose-dependently reduce the number of circulating lymphocytes in rats and mice. Additionally, in the experimental autoimmune encephalomyelitis (EAE) model in mice, Cenerimod was able to effectively improve disease-related parameters and alleviate symptoms. Therefore, Cenerimod holds promise as a new therapeutic agent with an improved safety profile for the treatment of autoimmune diseases, which have a significant medical need and currently lack effective treatment options.
Based on the results of phase II clinical trials, cenerimod not only reduces the migration of T cells and B cells but also diminishes the process by which professional antigen-presenting cells transport self-antigens to the lymph nodes, thereby reducing the presentation of self-antigens and the activation of lymphocytes. Additionally, cenerimod can decrease the levels of pro-inflammatory cytokines, thereby minimizing the inflammatory response. As a result, cenerimod will disrupt the vicious cycle involved in the pathogenesis of SLE. Therefore, the uniqueness of cenerimod lies in its simultaneous targeting of T cells, B cells, antigen-presenting cells, and the inflammation, offering an effective therapeutic approach for addressing multiple aspects of the pathogenesis in lupus.