Vigil Neuroscience: Phase 2 IGNITE trial shows promising early results for Iluzanebart (VGL101) in treating ALSP
Vigil Neuroscience, Inc., a company specializing in clinical-stage biotechnology, is dedicated to leveraging the potential of microglia for handling neurodegenerative diseases. The firm revealed promising preliminary results from their Phase 2 IGNITE proof-of-concept clinical trial, that is being conducted on patients suffering from adult-onset leukoencephalopathy with axonal spheroids and pigmented glia.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The initial information, depicting the initial six patients after half a year's treatment of 20 mg/kg iluzanebart (formerly referred to as VGL101), reinforces the beneficial safety and tolerability track record, as already observed in healthy volunteers. Additionally, these figures highlighted definite target interaction and subsequent pharmacological movement at 20 mg/kg, in line with the company’s past Phase 1 information from fit volunteers.
Beneficial trend changes were watched at half a year on magnetic resonance imaging and neurofilament light disease progression markers in unique ALSP patients. Ongoing findings from the Company's ongoing natural history examination, ILLUMINATE, continuously offer profound understanding into MRI and NfL biomarkers and backs soluble colony stimulating factor 1 receptor as a potential primary biomarker of ALSP disease pathology.
"The findings reflect the commitment and proficiency of our exceptional crew, who bring an unshakeable commitment to patients to their duties," concluded Dr. Magovčević-Liebisch. "To everyone who aided this advancement, including our trial members, their caretakers, and the clinical examiners, we give our deepest gratitude.
We anticipate revealing more information from the IGNITE trial, including results from all patients in both the 20 mg/kg and 40 mg/kg groups at half a year, in the third quarter of 2024."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
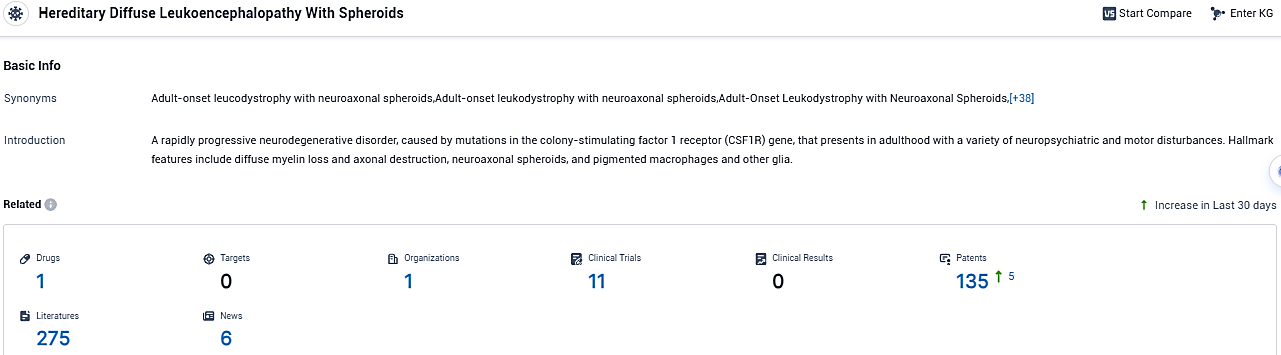
According to the data provided by the Synapse Database, As of November 26, 2023, there are 1 investigational drugs for the Hereditary Diffuse Leukoencephalopathy With Spheroids, 1 R&D institutions involved, with related clinical trials reaching 11, and as many as 135 patents.
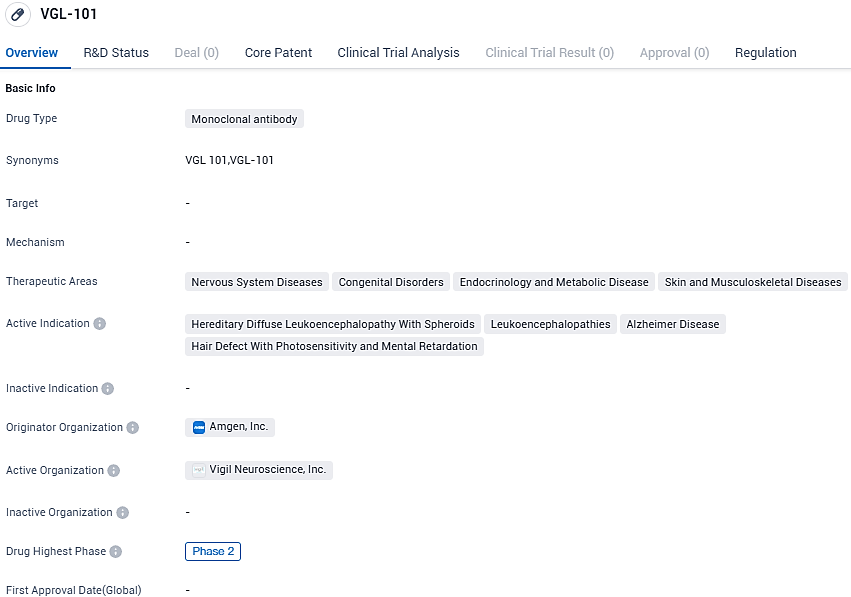
VGL-101 is intended to treat various conditions, including Hereditary Diffuse Leukoencephalopathy With Spheroids, Leukoencephalopathies, Alzheimer Disease, and Hair Defect With Photosensitivity and Mental Retardation. The drug has been designated as an Orphan Drug, indicating its potential to address rare diseases. Further research and regulatory processes are necessary to determine its effectiveness and safety.