Vir Biotechnology reports first subject treated in fresh Phase 1 trial of VIR-1388, a potential T cell HIV prevention vaccine under study
Vir Biotechnology, Inc. has reported that the first candidate has received a dosage in a Phase 1 trial, conducting an evaluation of the safety, reactogenicity, and immunogenicity of VIR-1388. This novel T cell vaccine is under investigation for the purpose of preventing Human Immunodeficiency Virus (HIV). The corporation predicts preliminary results from this Phase 1 study towards the latter half of 2024.
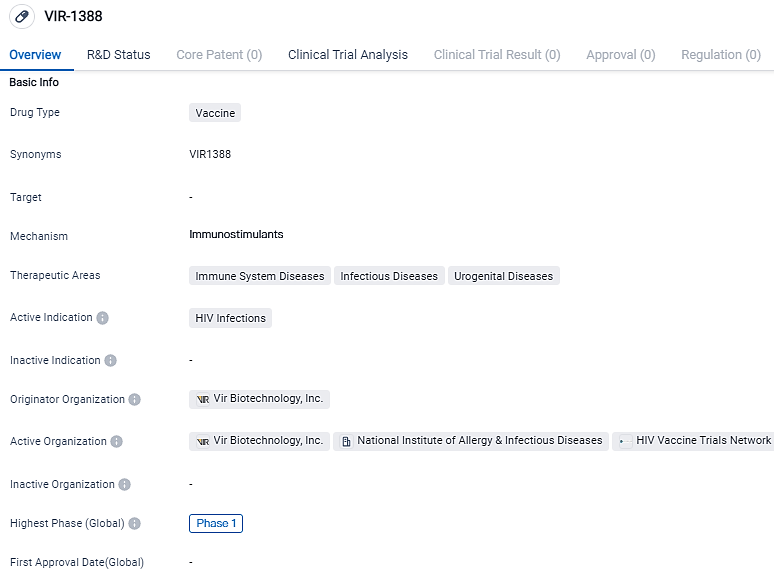
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
VIR-1388, a product engineered on the basis of the human cytomegalovirus vector platform, was designed to motivate the human body to create T cells, a type of immune cell, that respond to multiple HIV proteins in a unique manner, unlike former experimental HIV vaccines. The conceptualization and creation of VIR-1388 were influenced by findings from the development of VIR-1111, an initial experimental HIV T cell vaccine founded on HCMV, which was also a product of the company.
"Despite years of dedicated research, HIV remains a troubling cause of health issues globally as no endorsed vaccines exist," conveyed Dr. Carey Hwang, Vir's Senior Vice President for Clinical Research and Head of Chronic Infection. "Launching our first clinical trial aimed at evaluating VIR-1388 is a significant progression in our ambition to create an HIV vaccine. We appreciate the support offered by our partner organizations during this Phase 1 trial. We harbor optimism that our distinct strategy will help bridge the current public health discrepancy in HIV deterrence."
The development of VIR-1388 has been financially aided by the NIAID through its various stages and the Foundation has also backed the company in their development of treatments for HIV, prevention of malaria and tuberculosis. The Phase 1 trial of VIR-1388 is all set to be conducted across multiple national and international venues under the federally-sponsored HIV Vaccine Trials Network, known as study HVTN 142.
The majority of those who are infected with HCMV do not show any symptoms and are often unaware they carry the virus. It is understood that HCMV stays in the system for a lifetime, indicating its potential to convey and allow the body to retain HIV vaccine content for a prolonged duration. This could potentially help in overcoming the problems linked with the fading immunity seen with more ephemeral vaccine vectors.
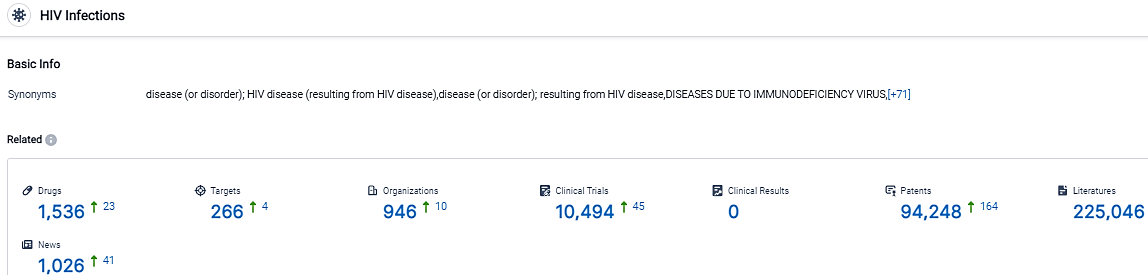
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
According to the data provided by the Synapse Database, As of September 26, 2023, there are 1536 investigational drugs for the HIV Infections, including 266 targets,946 R&D institutions involved, with related clinical trials reaching 10494,and as many as 94248 patents.
VIR-1388 is a developmental HIV T cell vaccine that is administered subcutaneously, and it is structured on HCMV. The vaccine is specially formulated to produce a large number of T cells that identify HIV proteins in a manner unique from previous experimental HIV vaccines. Information acquired from VIR-1111, Vir’s preliminary proof-of-concept HIV T cell vaccine, has been utilized when developing VIR-1388. The objective of this creation is to establish a secure and efficient HIV vaccine.