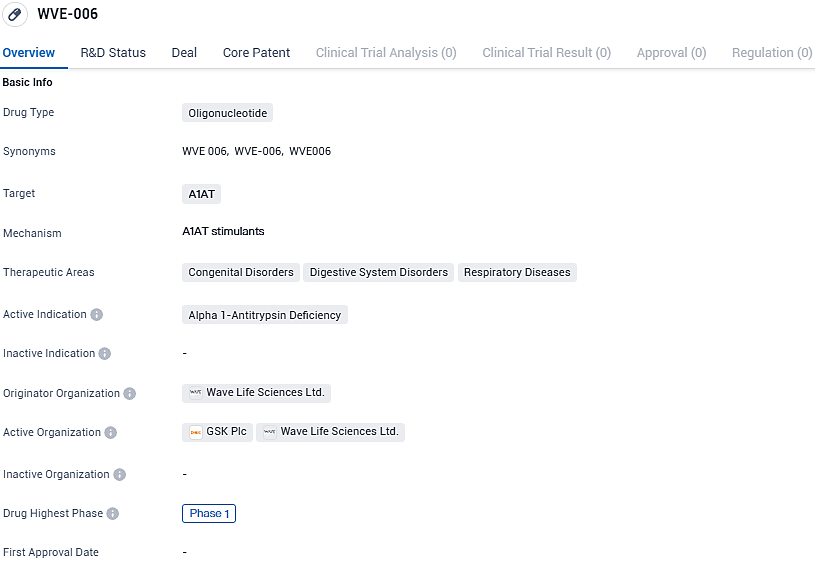
Wave Life Sciences initiated phase one of its RestorAATion trial to evaluate its innovative RNA therapy, WVE-006, targeting Alpha-1 Antitrypsin Deficiency
Wave Life Sciences Ltd., an enterprise focusing on RNA-based therapeutics in clinical development, is dedicated to developing transformative therapies for individuals grappling with severe health conditions. The company has recently declared the commencement of a dosing regimen for healthy participants within the scope of its RestorAATion clinical study agenda. This investigational endeavor examines the efficacy of WVE-006 as a promising therapy option for the genetic disorder known as alpha-1 antitrypsin deficiency (AATD).
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
WVE-006 represents a novel class of therapeutic agents, being a GalNAc-enhanced RNA editing oligonucleotide. Its function is to augment the presence of normal alpha-1 antitrypsin protein in the bloodstream while simultaneously curbing the accumulation of the defective Z-AAT protein. This dual action has the potential to target and mitigate complications of AATD that manifest in both the lungs and the liver.
"The commencement of dosing within the RestorAATion study is a significant event for individuals dealing with alpha-1 deficiency, a group that currently faces a dearth of treatment alternatives. Presently, no therapies directly target the predominant genetic aberration responsible for AATD," commented Paul Bolno, MD, MBA, who holds the positions of President and CEO at Wave Life Sciences.
"Furthermore, this occasion marks a pioneering venture into the realm of human therapeutics, as this is the maiden instance of an RNA editing drug being administered to patients. This symbolizes an influential step in the scientific arena, as we usher in a previously unexplored medication category into the sphere of clinical trials. We anticipate many more advances in RNA editing technology," expanded Bolno.
With the commencement of dosing through the RestorAATion program, Wave Life Sciences has met a significant early goal in its cooperative venture with GSK, an achievement that comes with a $20 million payment to Wave. The company may anticipate receiving up to an additional $505 million from GSK, which encompasses contingencies based on the progress of development, the launch and subsequent sales figures.
Additionally, Wave stands to gain staggered royalties on the net sales. Wave will remain responsible for development and market introduction duties until the RestorAATion-2 study is concluded, at which point GSK will assume these roles.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
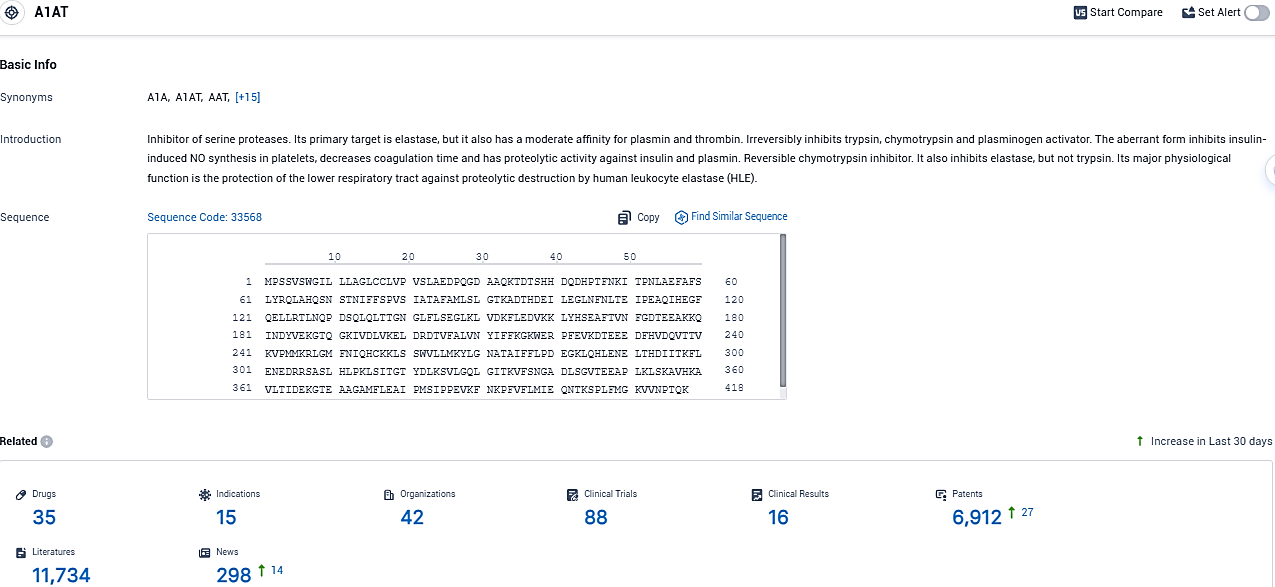
According to the data provided by the Synapse Database, As of December 13, 2023, there are 35 investigational drugs for the A1AT target, including 15 indications, 42 R&D institutions involved, with related clinical trials reaching 88, and as many as 6912 patents.
WVE-006 is a first-in-class, GalNAc-conjugated and subcutaneously administered RNA editing oligonucleotide designed to correct the single base mutation in messenger RNA coded by the SERPINA1 Z allele. In preclinical studies, WVE-006 demonstrated potent and durable editing of SERPINA1 Z transcript in mice, restoration of AAT protein up to 30 micromolar, and improvement in several markers of liver disease. WVE-006 is also highly specific with no evidence of bystander editing.