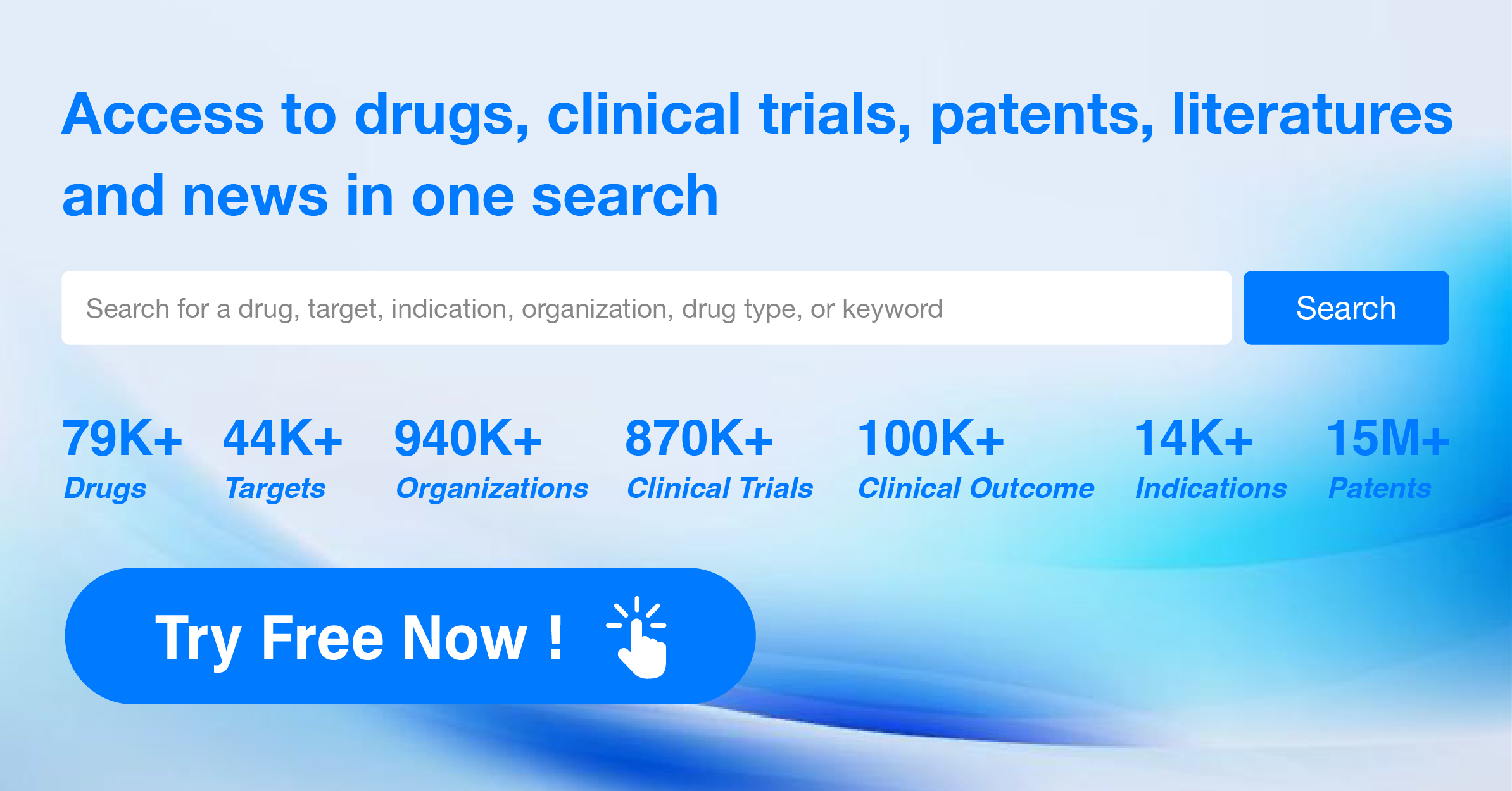
What information about clinical studies can be found in the ClinicalTrials.gov database?
The ClinicalTrials.gov database provides information about clinical trials that are currently recruiting or have recently been completed. It includes a variety of trial types, such as interventional, observational, prevention, treatment, diagnostic, device, behavioral, and education studies.
The database contains information on the trial's purpose, eligibility criteria, study design, location, sponsor, funding source, primary outcome measures, enrollment status, and estimated completion date. Additionally, it includes contact information for the trial's principal investigator(s), coordinator(s), and other relevant contacts.
To access the database, you can visit the website at https://clinicaltrials.gov/ and search for specific trials using keywords or filters such as trial type, disease, location, or sponsor.