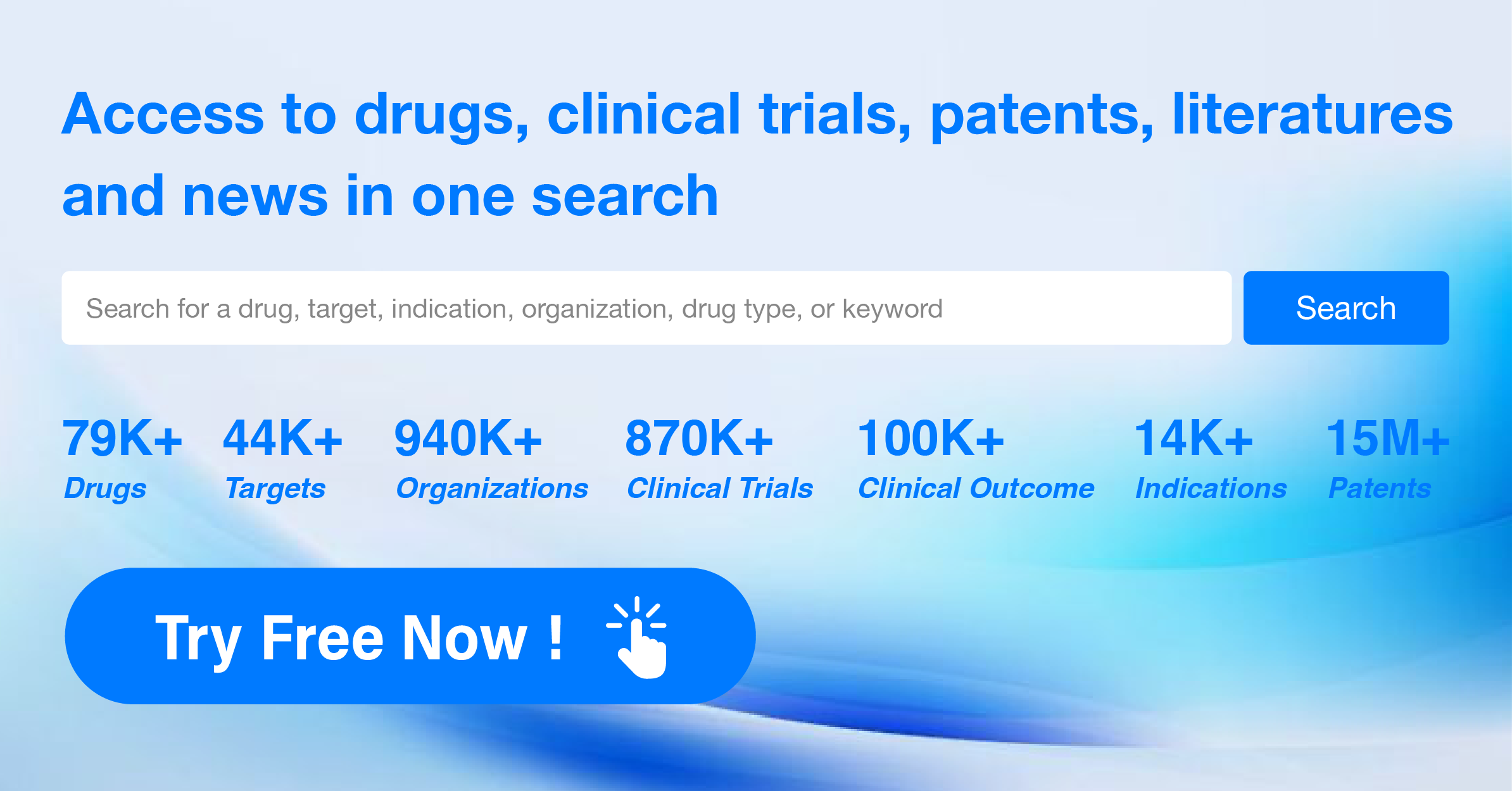
What is the difference between a generic drug and an innovator drug?
Generic drugs are medications that have been approved by the FDA as equivalent to brand-name drugs in terms of safety, efficacy, and quality. They are typically manufactured after the patent term for the innovator drug has expired or been challenged successfully.
In contrast, innovator drugs are new chemical entities (NCEs) that have not previously been approved by the FDA. These drugs often undergo extensive testing and research before being submitted for approval, and may receive a period of market exclusivity once they are approved. The exact length of this period varies depending on the type of drug and the specific circumstances of its development.
Regarding patent protection, the duration of exclusive rights granted to the innovator depends on various factors such as the type of drug, the extent of clinical trials conducted, and the submission process. For example, a small molecule with a well-characterized target may be eligible for 10 years of protection from the date of first approval. However, some biologic drugs can have longer periods of protection due to their complexity and the challenges involved in their development and production.