Xencor Initiates Phase 1/2 Trial of XmAb®942 in Inflammatory Bowel Disease
Xencor, Inc. (NASDAQ: XNCR), a biopharmaceutical company focused on clinical development of engineered antibodies targeting cancer and other critical illnesses, has announced that it has begun administering doses to healthy volunteers in the first human trial of XmAb®942, a novel high-potency, extended half-life anti-TL1A antibody. The company anticipates that initial results from this study will be available in the first half of 2025.
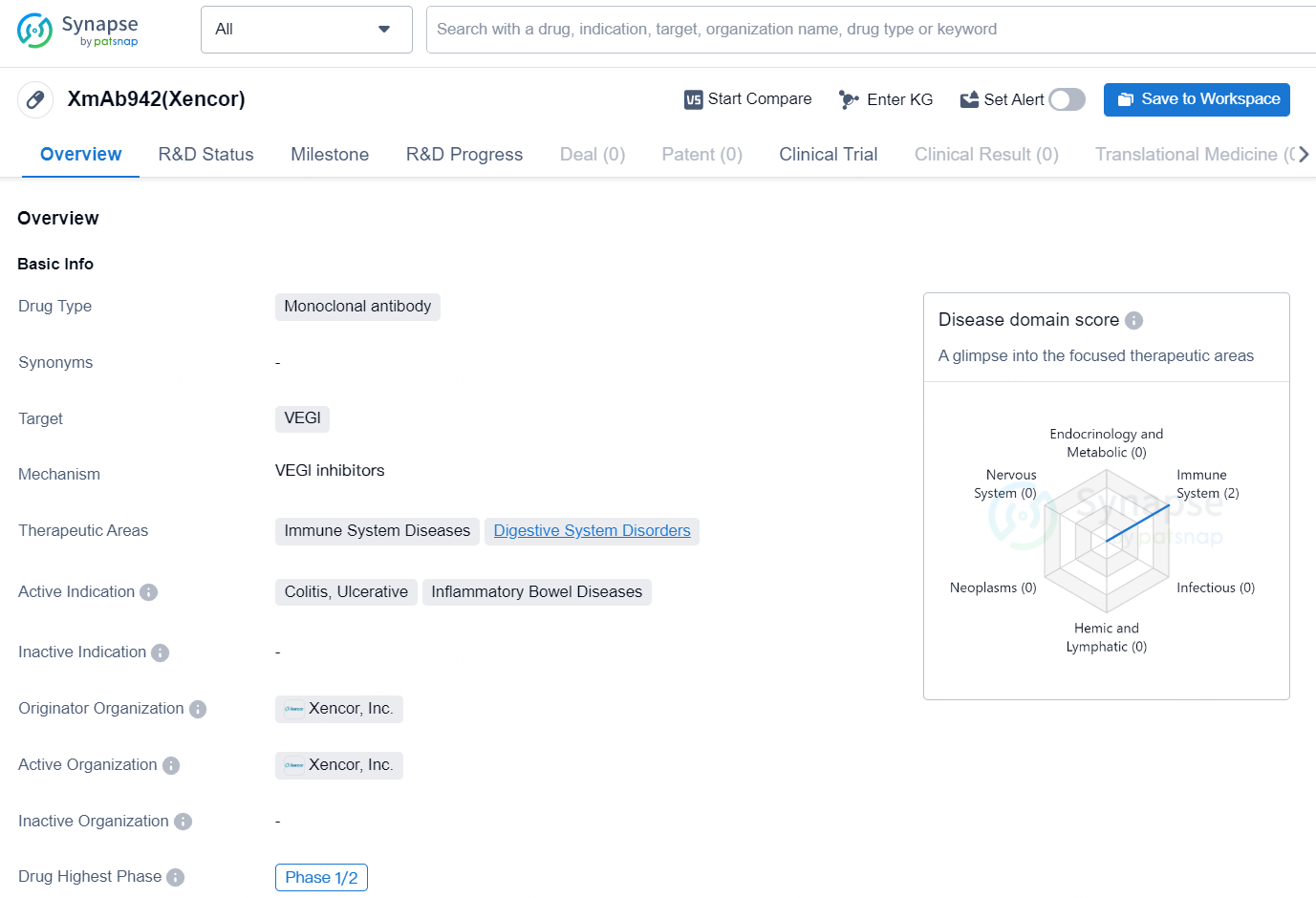
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"An engineered anti-TL1A antibody designed for enhanced target engagement and prolonged efficacy has the potential to significantly enhance treatment options for inflammatory bowel disease, stated Kenneth Hung, M.D., Ph.D., senior vice president of clinical development at Xencor. “We are thrilled to report the first administration of XmAb942 to healthy individuals and are optimistic that its characteristics, which may include leading potency within its class, could offer a therapeutic choice with enhanced clinical advantages and a more user-friendly dosing schedule compared to existing anti-TL1A antibodies in development.”
The Phase 1/2 trial of XmAb942 will be structured in three sections. In Phase 1, Part A will involve healthy participants in single-ascending dose (SAD) groups, while Part B will include additional healthy volunteers receiving multiple doses. For Phase 2, Part C will focus on patients with ulcerative colitis who will be administered the doses established in Parts A and B. (ClinicalTrials.gov Identifier: NCT06619990)
Xencor showcased a poster detailing the preclinical analysis of XmAb942 during the United Europe Gastroenterology Week (UEGW) in October 2024. The poster is accessible under the “Publications” and “Events & Presentations” sections on the Company’s website at www.xencor.com."
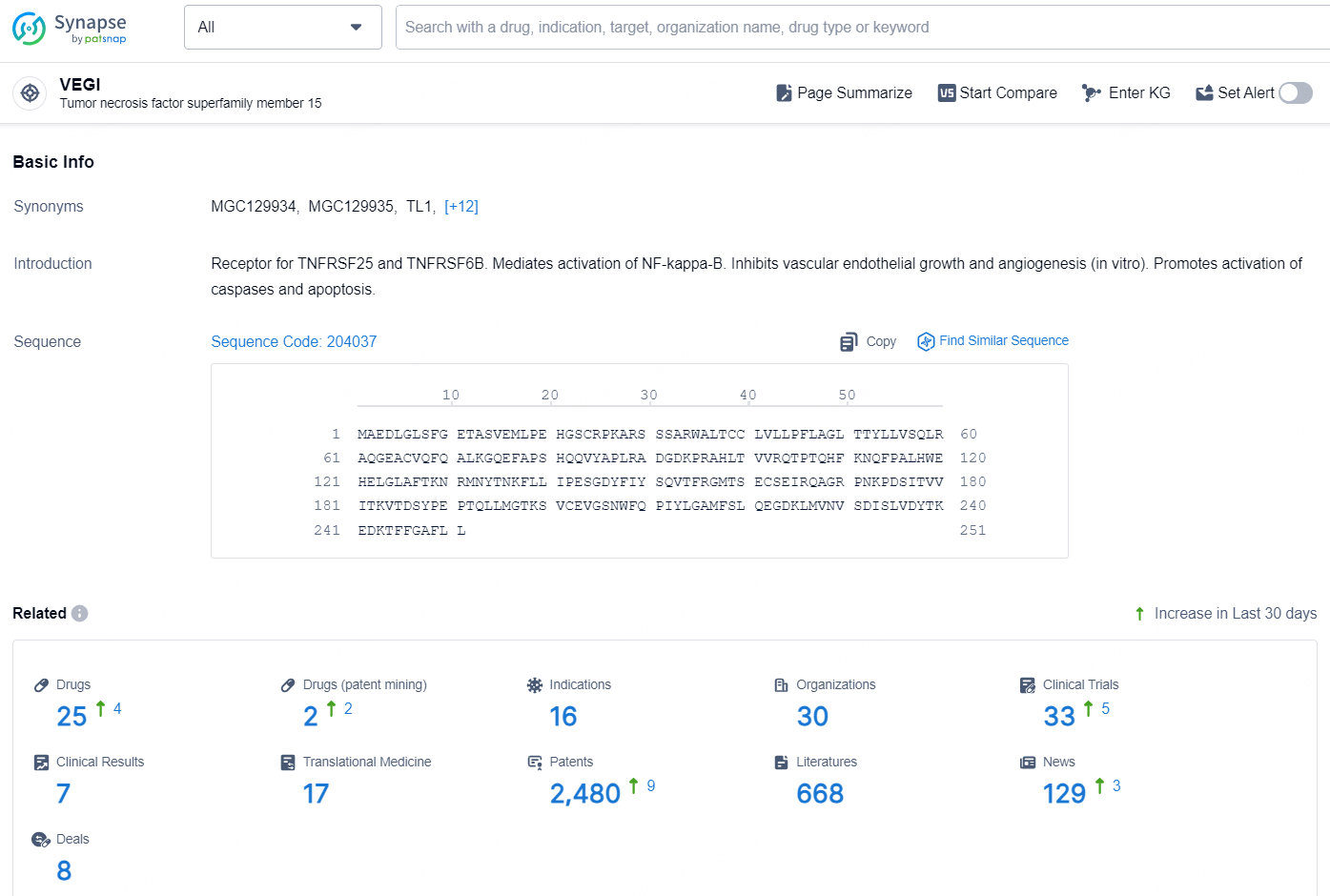
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of November 5, 2024, there are 25 investigational drug for the VEGI target, including 16 indications, 30 R&D institutions involved, with related clinical trials reaching 33, and as many as 2480 patents.
XmAb942(Xencor) is a monoclonal antibody drug developed by Xencor, Inc. The drug targets VEGI and is indicated for the treatment of colitis, ulcerative, and inflammatory bowel diseases. The therapeutic areas for for XmAb942(Xencor) include immune system diseases and digestive system disorders.