Zai Lab and argenx Secure China's Approval for Subcutaneous Efgartigimod Alfa in Generalized Myasthenia Gravis
Zai Lab Limited and argenx have announced that the Biologics License Application for efgartigimod alfa injection (efgartigimod SC), dosed at 1,000mg (5.6ml)/vial, received approval from China's National Medical Products Administration. This medication is indicated as an adjunct to standard treatment for adult patients with generalized myasthenia gravis who test positive for anti-acetylcholine receptor antibodies.
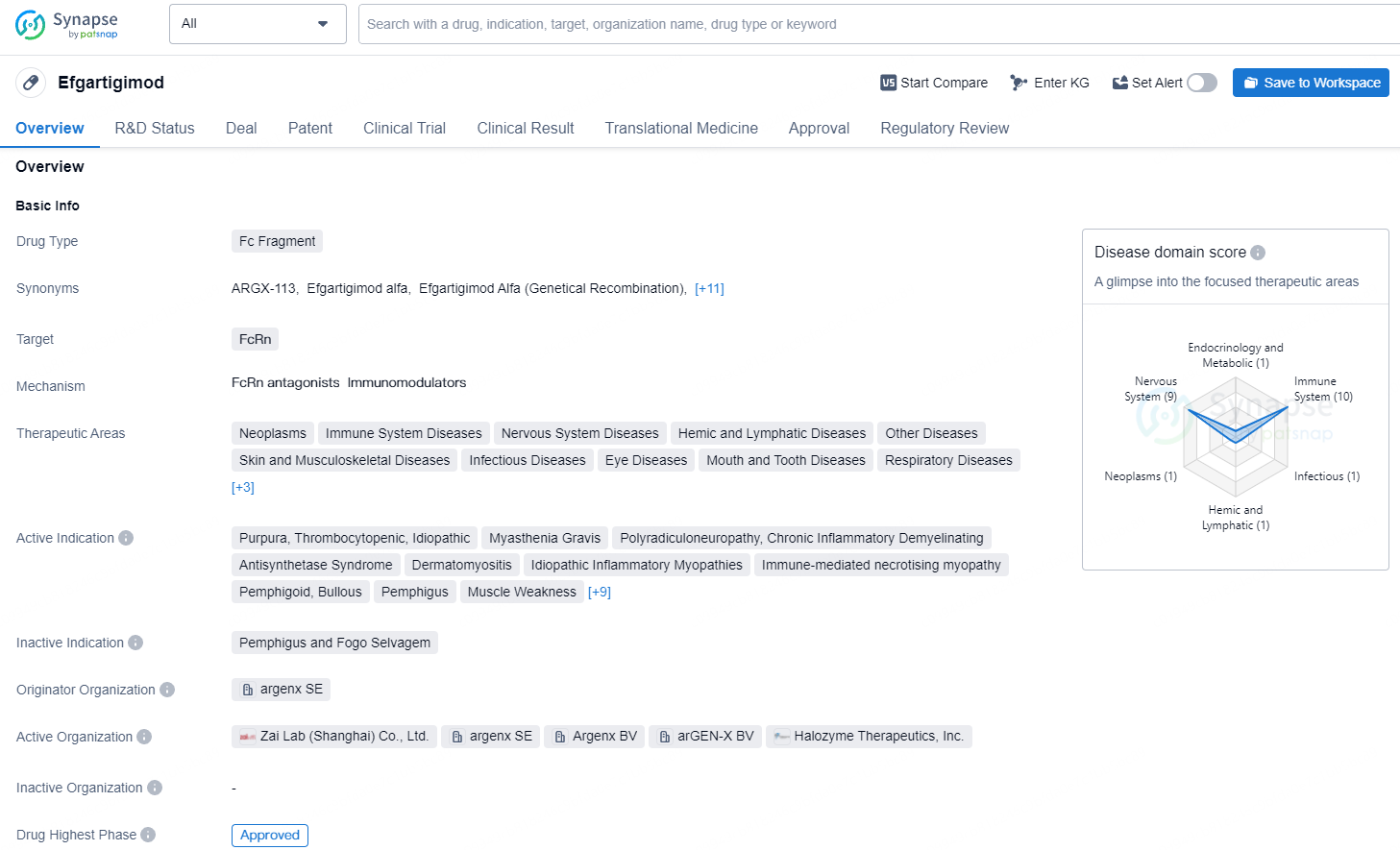
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
"We're excited to announce that efgartigimod SC has been granted approval by the NMPA, marking a significant achievement as we offer a pioneering treatment option for gMG patients in China," stated Rafael G. Amado, M.D., President and Head of Global Research and Development at Zai Lab. "Introducing a new therapeutic choice for gMG patients provides increased flexibility, potentially simplifying treatment regimens and enhancing community access to therapy. We are grateful to the NMPA for their comprehensive evaluation and acknowledgment of this therapy's distinct profile and the considerable unmet medical needs in China."
"The approval of efgartigimod SC by the NMPA is another critical milestone in our mission to extend our groundbreaking medication to new patient groups globally," said Tim Van Hauwermeiren, CEO of argenx.
"We share this accomplishment with our partner, Zai Lab, and we both hold a deep commitment to delivering essential innovations to gMG patients in China. We're impressed by Zai Lab's exceptional launch performance, having introduced 2,700 new patients to VYVGART IV treatment in the first quarter of 2024, demonstrating the substantial unmet need among gMG patients," added Tim Van Hauwermeiren.
The safety profile associated with efgartigimod SC aligns with the findings from the ADAPT study. Efgartigimod SC was generally well-tolerated, with the most common adverse event being injection site reactions, a typical occurrence with subcutaneously administered biologics. All ISRs were mild to moderate and resolved over time.
Additionally, efgartigimod SC is under evaluation for treating other autoimmune disorders. In May 2024, the NMPA accepted a supplemental Biologics License Application with priority review for efgartigimod SC in chronic inflammatory demyelinating polyneuropathy. The FDA approved efgartigimod SC in June 2024 for adult patients with CIDP.
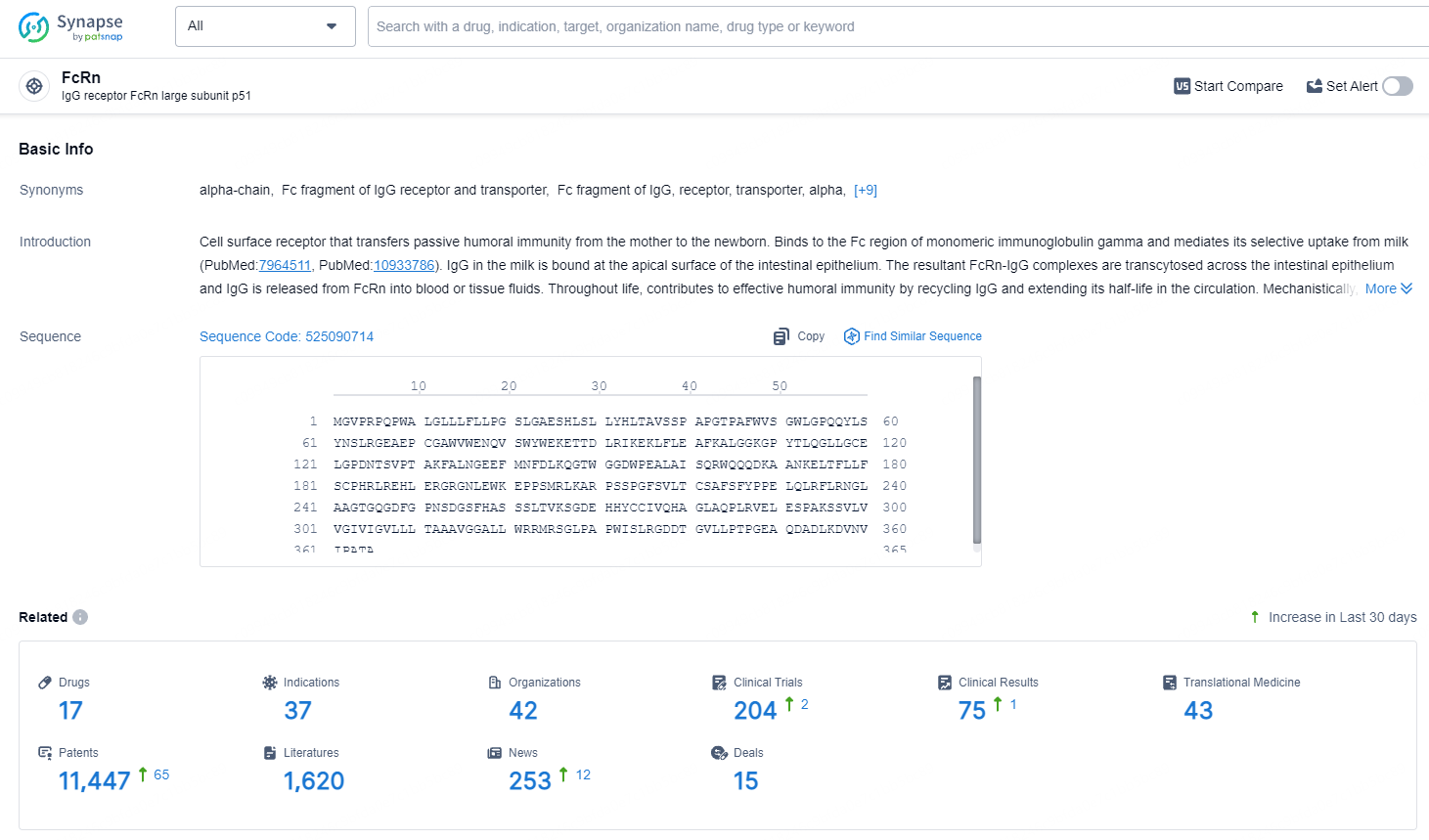
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 18, 2024, there are 17 investigational drugs for the FcRn target, including 37 indications, 42 R&D institutions involved, with related clinical trials reaching 204, and as many as 11447 patents.
Efgartigimod is a Fc fragment drug that targets the FcRn. Efgartigimod has emerged as a promising addition to the pharmaceutical landscape, with its approval marking a significant milestone in the treatment of various immune-mediated conditions. Its wide range of therapeutic areas and active indications underscore its potential to benefit patients across different disease categories, providing new avenues for managing complex and challenging medical conditions.