Zenocutuzumab: A Quick Look at Its R&D Progress and Clinical Results from the 2023 ESMO_ASIA
NRG1 fusions are rare oncogenic drivers of NSCLC and other solid tumors. These chimeric proteins bind HER3, leading to HER2/HER3 heterodimerization and oncogenic transformation. Zenocutuzumab (MCLA-128; Zeno) is a bispecific antibody that overcomes HER3-mediated NRG1 signaling in NRG1+ cancer. Zeno is being evaluated in the ongoing pivotal phase 2 eNRGy study and early access program (EAP). Updated NRG1+ NSCLC data are presented in 2023 ESMO_ASIA.
Zenocutuzumab's R&D Progress
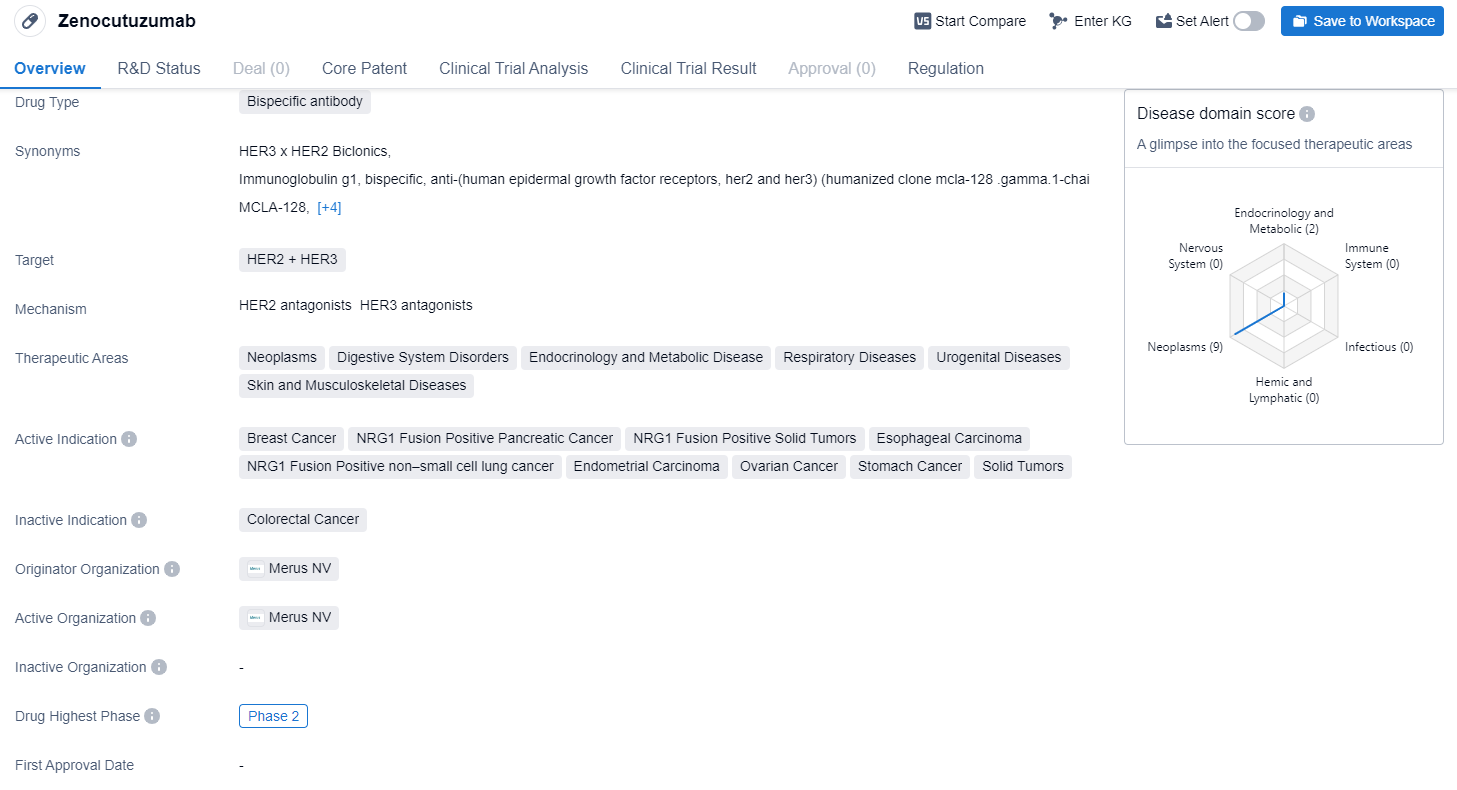
Zenocutuzumab is a bispecific antibody drug that targets HER2 and HER3 receptors. The therapeutic areas that Zenocutuzumab aims to address include neoplasms (abnormal growth of cells), digestive system disorders, endocrinology and metabolic diseases, respiratory diseases, urogenital diseases, and skin and musculoskeletal diseases. The drug is indicated for various types of cancers, including breast cancer, NRG1 fusion positive pancreatic cancer, NRG1 fusion positive solid tumors, esophageal carcinoma, NRG1 fusion positive non-small cell lung cancer, endometrial carcinoma, ovarian cancer, stomach cancer, and solid tumors.
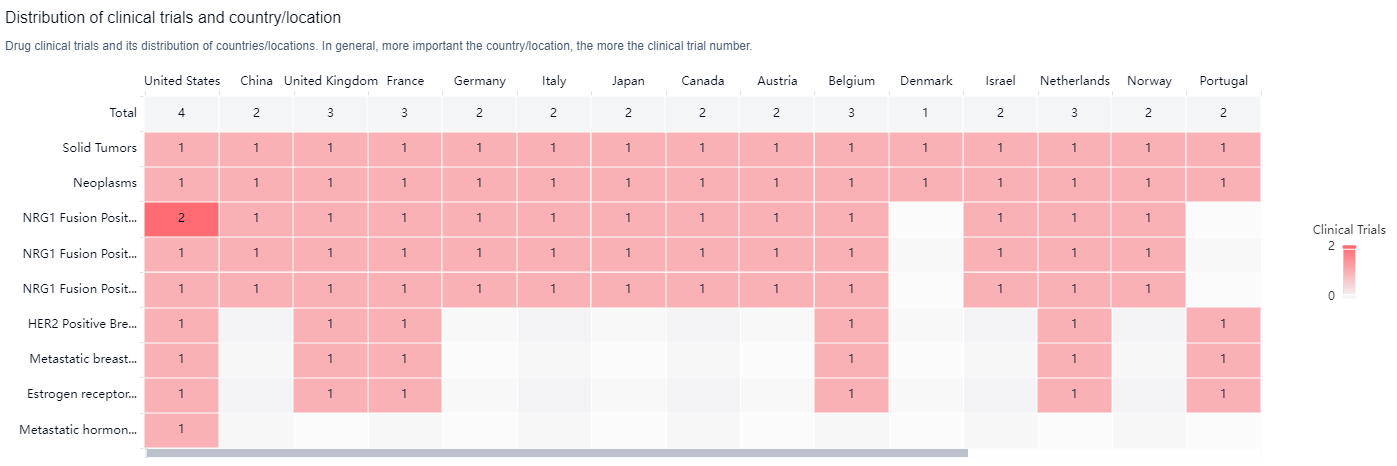
According to the Patsnap Synapse, Zenocutuzumab is being developed by Merus NV and has reached Phase 2 of clinical trials globally. In China, it has received IND (Investigational New Drug) approval. And the clinical trial distributions for Zenocutuzumab are primarily in the United States, China and United Kingdom. The key indication is Solid Tumors.
Detailed Clinical Result of Zenocutuzumab
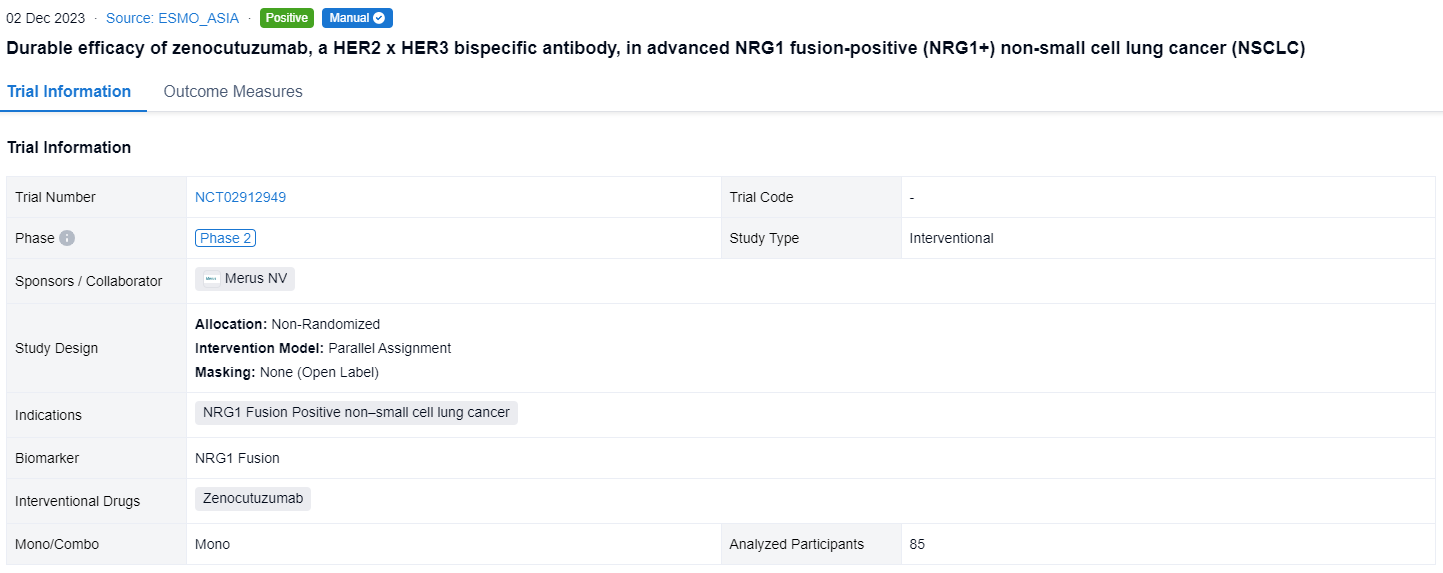
This non-randomized, parallel assignment, open-labeled clinical trial (NCT02912949) was aimed to explore the efficacy and safety of Zenocutuzumab in advanced NRG1 fusion-positive (NRG1+) non-small cell lung cancer (NSCLC).
In this study, patients (pts) with advanced NRG1+ NSCLC determined by NGS, previously treated with or not candidate for standard therapy, age ≥ 18 y, ECOG PS ≤ 2, and measurable (RECIST v1.1) or evaluable disease were enrolled. Zeno (750 mg IV Q2W) was administered until disease progression or unacceptable toxicity. Tumor imaging was conducted Q8W. The primary endpoint is investigator-assessed objective response rate (ORR) per RECIST v1.1. Secondary endpoints include duration of response (DOR) and safety.
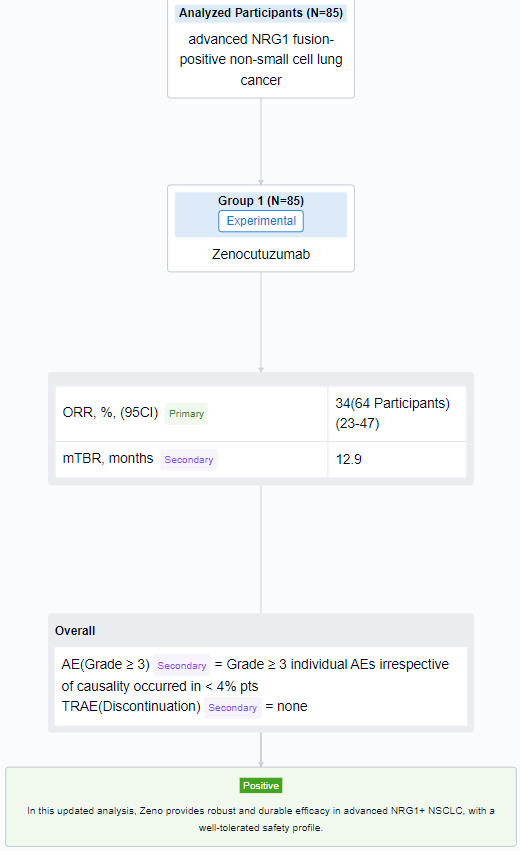
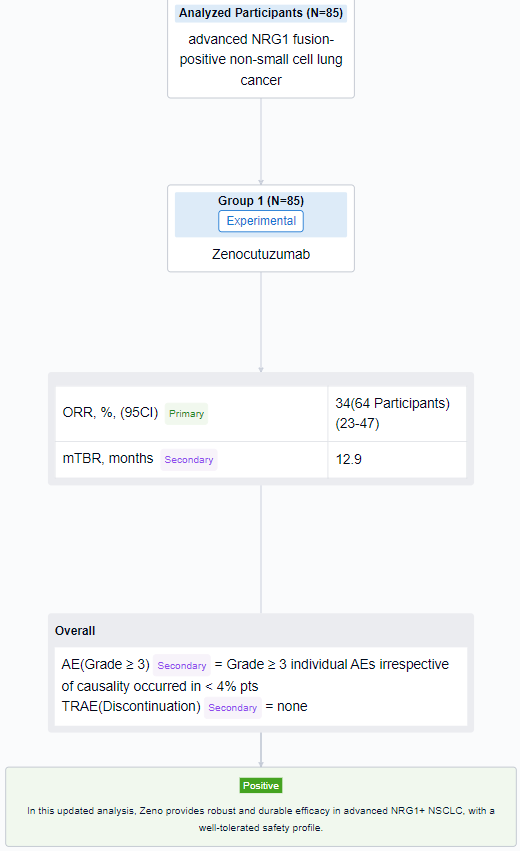
The result showed that as of 01 Feb 2023, 85 pts with NRG1+ NSCLC (78 eNRGy/7 EAP) were enrolled. Efficacy was assessed in 65 pts who received ≥ 1 dose of Zeno and were enrolled by 01 Aug 2022 to allow for the opportunity for ≥ 6 months (mo) follow-up and met the criteria for the primary efficacy population. The median age was 64 y (range 32–86), 65% were female, 29%/63%/5% pts had ECOG PS 0/1/2 (missing 2 pts). Most were Asian (52%) or White (37%), 77% had visceral metastases, 98% had adenocarcinoma histology, and 1 pt had non-measurable disease. Common fusion partners were CD74 (52%) and SLC3A2 (23%). Pts received a median of 2 prior systemic therapies (range 0-6), 78% with platinum-based chemotherapy; 12% were treatment naïve. In the 64 pts with measurable disease, the confirmed ORR was 34% (22/64; 95% CI 23-47). 50/64 (78%) pts had target lesion reduction. Median DOR was 12.9 mo with responses ongoing in 11/22 (50%) pts. Kaplan-Meier estimate of 6-mo DOR rate was 79%. Among 85 pts treated with Zeno, Grade ≥ 3 individual AEs irrespective of causality occurred in < 4% pts. No pt discontinued Zeno for a treatment related AE.
It can be concluded that in this updated analysis, Zeno provides robust and durable efficacy in advanced NRG1+ NSCLC, with a well-tolerated safety profile.
How to Easily View the Clinical Results Using Synapse Database?
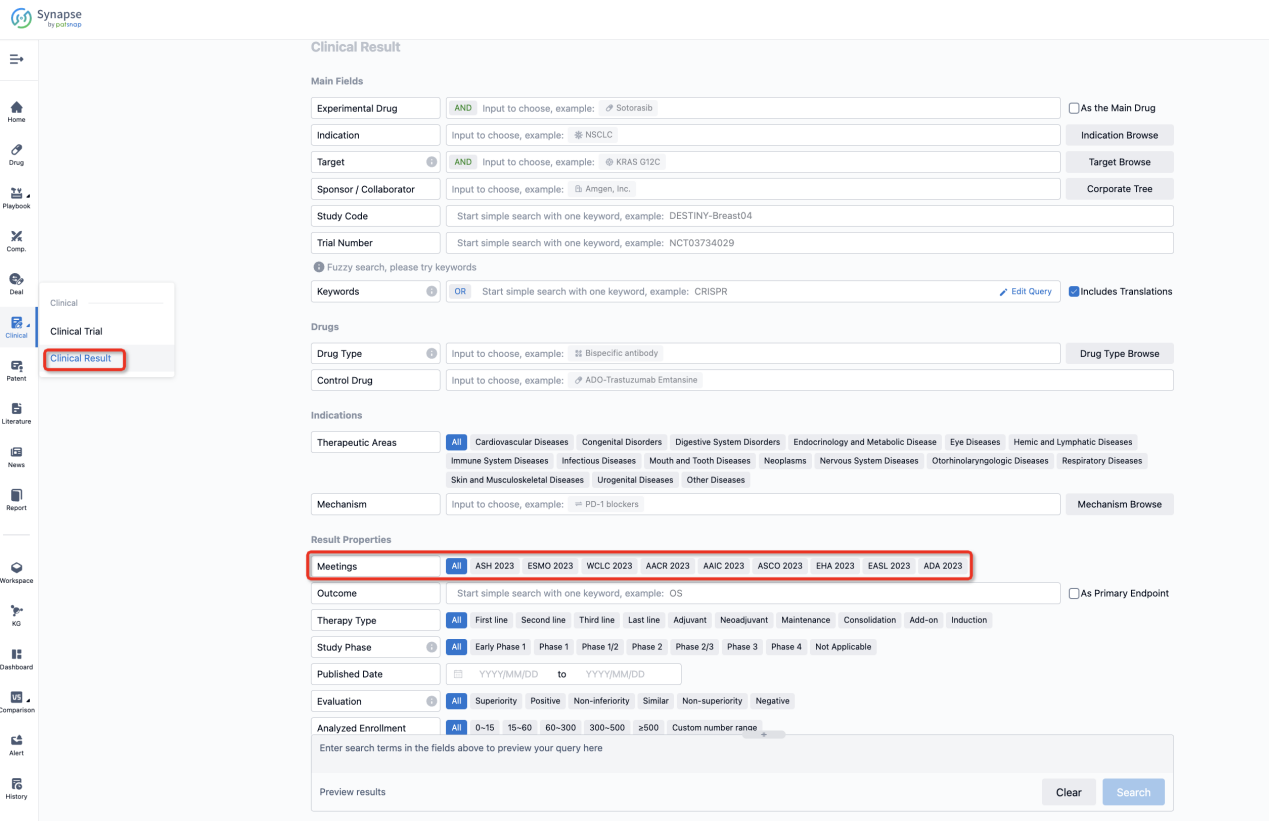
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
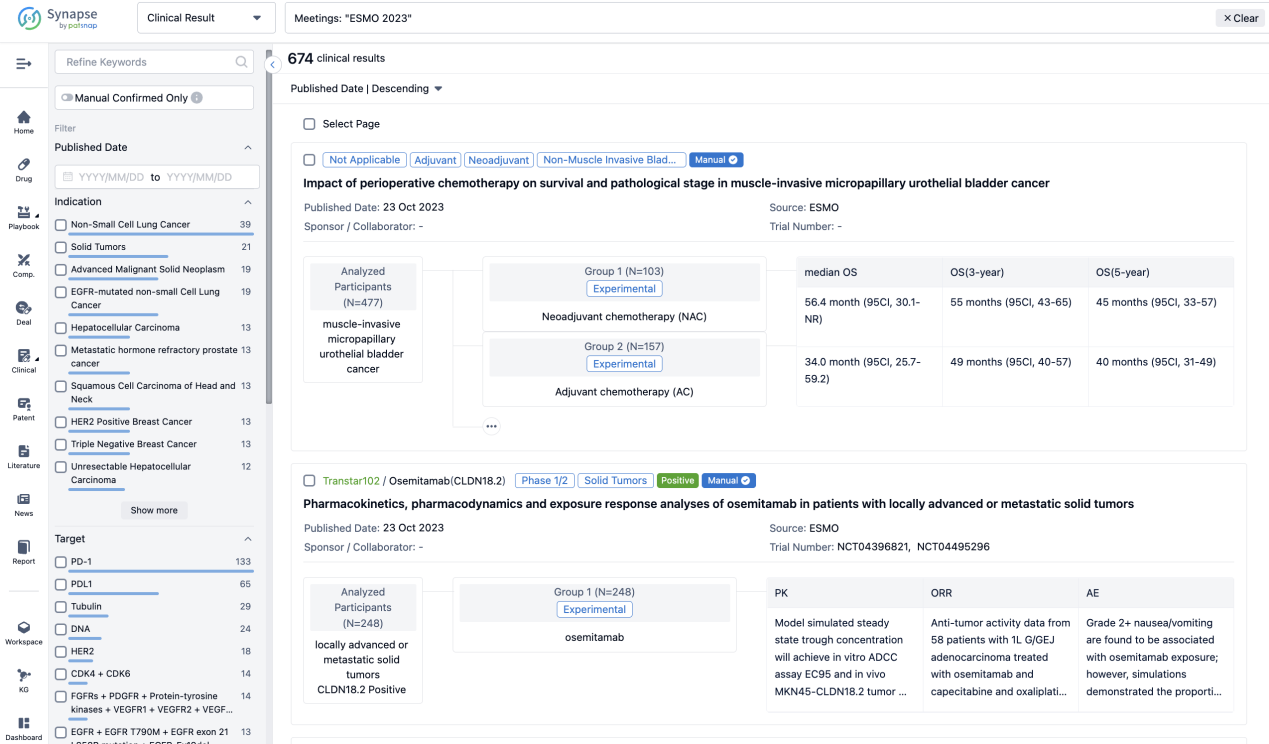
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
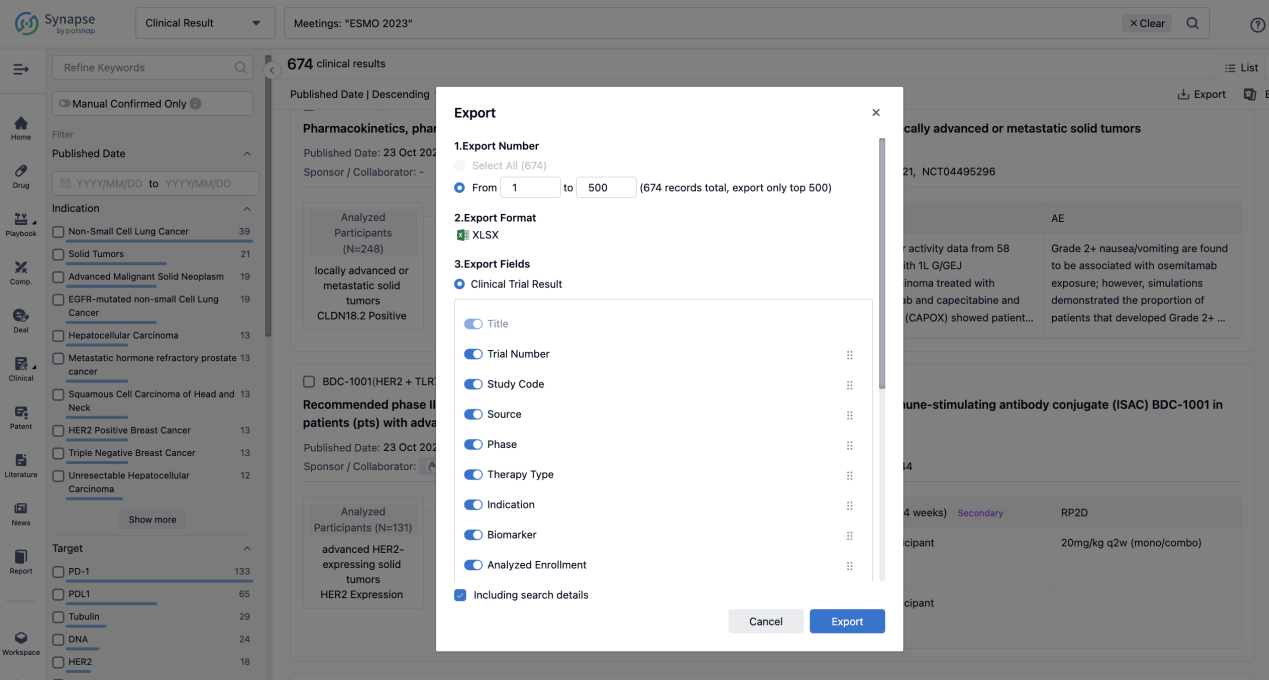
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!