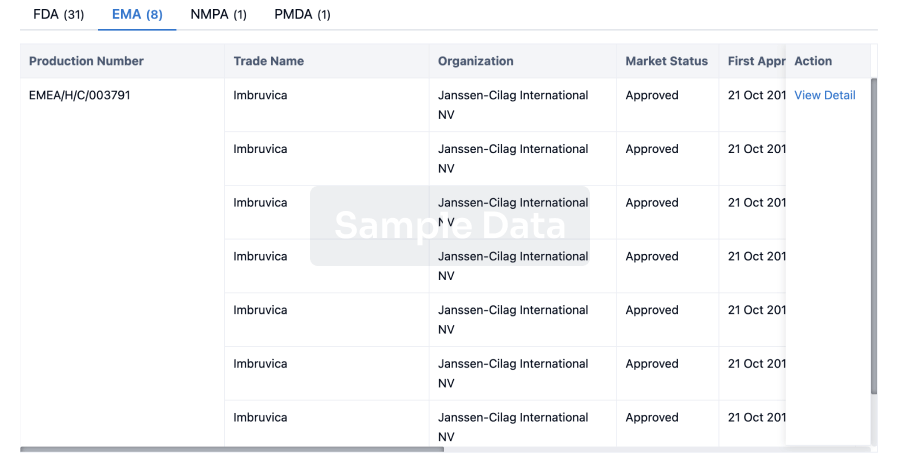
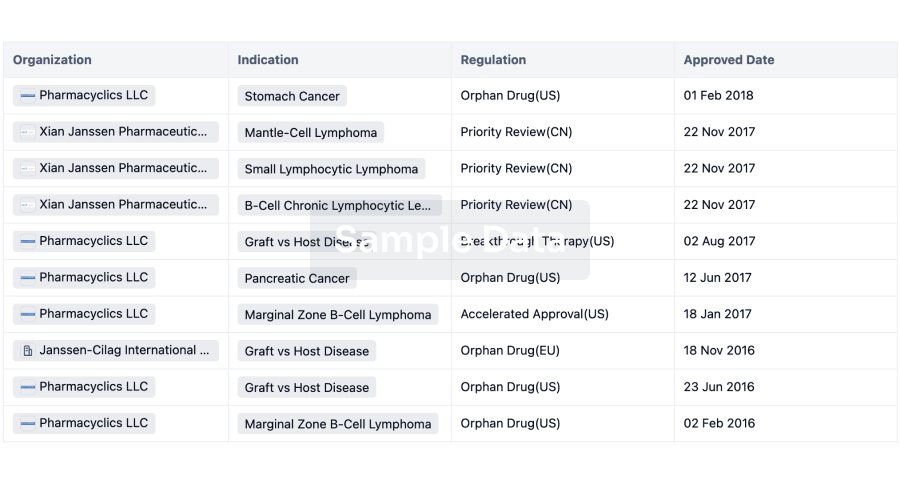
RALEIGH, N.C., April 8, 2025 /PRNewswire/ -- GeneVentiv Therapeutics, a gene therapy company, today announced it has signed a global licensing agreement with Duke University for the first universal gene editing therapy for both infantile-onset and late-onset Pompe disease, which affect 120,000 patients in the developed world. The therapy has been designated GENV-002.
Continue Reading
Dr. Dwight Koeberl (left), Professor of Pediatrics at Duke University, and Damon Race (right), CEO of GeneVentiv Therapeutics, shake hands to celebrate the signing of GeneVentiv's license agreement for Dr. Koeberl's Universal Gene Editing Therapy for Infantile Onset (IOPD) and Late Onset (LOPD) Pompe disease.
Pompe disease results from a deficiency of an enzyme called acid-alpha-glucosidase (GAA), leading to massive lysosomal glycogen buildup in striated muscle, resulting in respiratory failure, limited mobility, persistent muscle weakness, and heart complications, severely restricting daily activities.
Current treatment places a heavy burden on families. Enzyme Replacement Therapy (ERT) requires biweekly infusions at specialized centers, lasting 4-6 hours and costing up to $11.7 million over a lifetime. While ERT has extended survival in infantile-onset cases, no curative therapy exists.
GENV-002 was invented by Dwight Koeberl, M.D., Ph.D., professor in the Department of Pediatrics and a medical genetics specialist at Duke University, along with leading experts in genetic disease and specifically Pompe disease. Dr. Koeberl previously invented a gene therapy limited to treating late-onset Pompe disease that was licensed to AskBio.
For nearly two decades, Dr. Koeberl has been working toward a treatment that could overcome the inability of adeno-associated virus (AAV) gene therapies to treat all infants with Pompe disease. GENV-002 is a liver depot strategy utilizing AAV for delivery of a healthy donor gene and a CRISPR Cas9 editor for gene integration.
The culmination of Dr. Koeberl's work is GENV-002. Combined with newborn screening for both infantile- and late-onset Pompe disease, GENV-002 can be administered to all patients as infants, a decade or more in advance of non-genome editing AAV competitors. The technology was licensed through the Duke University Office for Translation & Commercialization.
"GeneVentiv has made significant strides in executing our business model of identification of transformative gene therapies, in-licensing and development," said Damon Race, CEO of GeneVentiv Therapeutics. "The therapies we have licensed share a common theme as universal therapies that are the first to address patients left behind by the shortcomings of other approaches. With our latest license we can now address greater than 270,000 developed-world patients with a pricing precedent of $3 million for a single infusion gene therapy."
"Our lead program GENV-HEM is a universal gene therapy for all hemophilias and the first to durably address hemophilia A with or without inhibitors, which Factor VIII gene therapies could not achieve," said Paris Margaritis, CEO of GeneVentiv Therapeutics. "Similarly, GENV-002 surpasses existing AAV gene therapies with the ability to universally treat infantile- and late-onset Pompe disease in infancy via gene editing."
About GeneVentiv Therapeutics:
GeneVentiv Therapeutics is a pre-clinical gene therapy focused on identifying, in-licensing and developing transformative gene therapies. GeneVentiv has two first-in-class gene therapies for patients with significant unmet needs. GENV-HEM (AAV8.FVa) is a gene therapy for all hemophilias, including inhibitor patients, and without the durability issues that have plagued Factor VIII gene therapies. GENV-002 (AAV9.GAA and AAV9.Cas9-gRNA) is a first-in-class gene editing therapy for both the infantile- and late-onset forms of Pompe disease that stably integrates a functional GAA transgene enabling treatment very early in life with stable expression, something that other gene therapies are unable to achieve. With these gene therapies we can transform the lives of 270,000+ developed world patients. Explore, learn more and grow with us by visiting .
Media Contact:
Damon Race
[email protected]
(919) 529-3352
SOURCE GeneVentiv Therapeutics, Inc.
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
440k+
Newsrooms &
Influencers
9k+
Digital Media
Outlets
270k+
Journalists
Opted In
GET STARTED