Request Demo
Last update 18 Jul 2025
Fidrisertib succinate
Last update 18 Jul 2025
Overview
Basic Info
Drug Type Small molecule drug |
Synonyms BLU 782, BLU-782, BLU-782 sesquisuccinate + [2] |
Target |
Action inhibitors |
Mechanism ALK2 inhibitors(Activin receptor type-1 inhibitors) |
Active Indication |
Inactive Indication- |
Originator Organization |
Active Organization |
Inactive Organization |
License Organization |
Drug Highest PhasePhase 2 |
First Approval Date- |
RegulationOrphan Drug (Japan) |
Login to view timeline
Structure/Sequence
Molecular FormulaC31H42N6O4 |
InChIKeySWVYYNLRVIYURK-AREMUKBSSA-N |
CAS Registry2141955-96-4 |
Related
3
Clinical Trials associated with Fidrisertib succinateNCT05039515
A Phase 2 Study to Assess the Efficacy and Safety of 2 Dosage Regimens of Oral Fidrisertib (IPN60130) for the Treatment of Fibrodysplasia Ossificans Progressiva in Male and Female Paediatric and Adult Participants.
Fibrodysplasia Ossificans Progressiva (FOP) is a rare, severely disabling disease characterized by the presence of bone in soft tissue where bone normally does not exist, known as Heterotopic Ossification (HO). It is often associated with painful, recurrent episodes of soft tissue swelling (flare-ups) that lead to abnormal stiffening and immobility (ankyloses) of major joints with cumulative and irreversible loss of movement and disability.
This study will evaluate the efficacy of 2 dosing regimens of IPN60130 in inhibiting new HO volume compared with placebo (a dummy treatment) in adult and paediatric participants with FOP. It will be assessed by a scan (provides internal images of the body) called low dose Whole Body Computed Tomography (WBCT), excluding head.
Adults and participants 5 years of age or older are also eligible for a sub study to evaluate HO lesions assessed by another type of scan, Fluorine-18-labelled natrium fluoride Positron Emission Tomography-Computed Tomography ([18F]NaF PET-CT ).
This study will evaluate the efficacy of 2 dosing regimens of IPN60130 in inhibiting new HO volume compared with placebo (a dummy treatment) in adult and paediatric participants with FOP. It will be assessed by a scan (provides internal images of the body) called low dose Whole Body Computed Tomography (WBCT), excluding head.
Adults and participants 5 years of age or older are also eligible for a sub study to evaluate HO lesions assessed by another type of scan, Fluorine-18-labelled natrium fluoride Positron Emission Tomography-Computed Tomography ([18F]NaF PET-CT ).
Start Date01 Dec 2021 |
Sponsor / Collaborator |
NL-OMON50811
A Phase 1, open-label, single-dose, single-center, mass balance study of IPN60130 following a single 60-mg oral dose of IPN60130 containing 9 µCi of [14C]-IPN60130 in healthy male participants - ADME Study of 14C-IPN60130 in 6 healthy male subjects
Start Date28 Jul 2021 |
Sponsor / Collaborator |
NCT03858075
A Phase 1, Randomized, Double-Blind, Placebo-Controlled, Single and Multiple Ascending Dose and Food Effect Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of BLU-782 When Administered Orally to Healthy Adult Subjects
The main objectives of this study are to assess the safety, tolerability, pharmacokinetics (PK), and food effect of BLU-782 in healthy adult subjects.
Start Date12 Feb 2019 |
Sponsor / Collaborator |
100 Clinical Results associated with Fidrisertib succinate
Login to view more data
100 Translational Medicine associated with Fidrisertib succinate
Login to view more data
100 Patents (Medical) associated with Fidrisertib succinate
Login to view more data
1
Literatures (Medical) associated with Fidrisertib succinate29 May 2024·Science Translational Medicine
An ALK2 inhibitor, BLU-782, prevents heterotopic ossification in a mouse model of fibrodysplasia ossificans progressiva
Article
Author: Lobbardi, Riadh ; Fleming, Paul ; Kadambi, Vivek ; Russo, Joelle ; Hussein, Amira ; Palmer, Michael ; Lyon, Morgan ; Graul, Chris ; Dorsch, Marion ; Wilson, Douglas ; Davis, Alison J. ; Zhu, Xing Julia ; Xu, Lan ; Albayya, Faris ; Hodous, Brian L. ; Bouchard, Keith ; Guzi, Timothy J. ; Vassiliadis, John ; Brooijmans, Natasja ; Williams, Brett D. ; Stevison, Faith ; Garner, Andrew P. ; Greenblatt, Elliot ; Brubaker, Jason D. ; Lengauer, Christoph ; Sheets, Michael P. ; Hunter, Jeffrey W. ; Kim, Joseph L. ; Wang, Ruduan ; LaBranche, Timothy P. ; Kim, Sean ; Stewart, Rachel
Fibrodysplasia ossificans progressiva (FOP) is a rare genetic disease driven by gain-of-function variants in activin receptor–like kinase 2 (ALK2), the most common variant being ALK2
R206H
. In FOP, ALK2 variants display increased and dysregulated signaling through the bone morphogenetic protein (BMP) pathway resulting in progressive and permanent replacement of skeletal muscle and connective tissues with heterotopic bone, ultimately leading to severe debilitation and premature death. Here, we describe the discovery of BLU-782 (IPN60130), a small-molecule ALK2
R206H
inhibitor developed for the treatment of FOP. A small-molecule library was screened in a biochemical ALK2 binding assay to identify potent ALK2 binding compounds. Iterative rounds of structure-guided drug design were used to optimize compounds for ALK2
R206H
binding, ALK2 selectivity, and other desirable pharmacokinetic properties. BLU-782 preferentially bound to ALK2
R206H
with high affinity, inhibiting signaling from ALK2
R206H
and other rare FOP variants in cells in vitro without affecting signaling of closely related homologs ALK1, ALK3, and ALK6. In vivo efficacy of BLU-782 was demonstrated using a conditional knock-in ALK2
R206H
mouse model, where prophylactic oral dosing reduced edema and prevented cartilage and heterotopic ossification (HO) in both muscle and bone injury models. BLU-782 treatment preserved the normal muscle-healing response in ALK2
R206H
mice. Delayed dosing revealed a short 2-day window after injury when BLU-782 treatment prevented HO in ALK2
R206H
mice, but dosing delays of 4 days or longer abrogated HO prevention. Together, these data suggest that BLU-782 may be a candidate for prevention of HO in FOP.
7
News (Medical) associated with Fidrisertib succinate16 Apr 2025
Total sales growth of 11.6% at CER1, or 11.7% as reported, driven by all three therapeutic areas and including an increasing contribution from Iqirvo and Bylvay. Tovorafenib regulatory submission to EMA2 for pediatric low-grade glioma. Confirmation of full-year 2025 financial guidance.
PARIS, FRANCE, 16 April 2025 – Ipsen (Euronext: IPN; ADR: IPSEY), a global specialty-care biopharmaceutical company, today presents sales for the first quarter of 2025.
“Ipsen has delivered a strong start to 2025, building further momentum in the transformation of our company,” commented David Loew, Chief Executive Officer, Ipsen. “We continued to execute on our strategy with strong top-line growth and pipeline progression. I am pleased to see the rapid build-up of our Rare Cholestatic Liver disease franchise with two innovate medicines for five indications. 2025 is set to be an important year for Ipsen, with multiple launches underway and several milestones expected across our portfolio.”
Full-year guidance
Ipsen is confirming financial guidance for full-year 2025:
Total sales growth greater than 5.0%, at constant currency. Based on the average level of exchange rates in March 2025, a limited effect on total sales from currencies is expected. Core operating margin greater than 30.0% of total sales, which includes additional R&D expenses from anticipated early and mid-stage external-innovation opportunities.
Guidance includes expected negative impact on Somatuline sales due to increased generic competition in the U.S. and Europe. It excludes any impact from potential late-stage (Phase III clinical development or later) business development transactions.
Upcoming Milestones
Ipsen anticipates several key milestones across its portfolio in 2025, including:
Cabometyx (CABINET trial) – Regulatory decision in the European Union for advanced pancreatic (pNETs) and extra-pancreatic (epNETs) neuroendocrine tumors (NETs). fidrisertib (FALKON trial) – Readout of the pivotal Phase IIb trial in fibrodysplasia ossificans progressiva (FOP). LANT3 (LANTIC trial) – Proof-of-concept data readout, evaluating its potential in aesthetics.
Q1 pipeline progress
The regulatory filing for tovorafenib was accepted by EMA for review in the European Union, marking an important step forward in the development of this potential treatment for pediatric low-grade glioma and reinforcing Ipsen’s commitment to innovation in rare and difficult-to-treat cancers.
Ipsen also initiated the entry in Phase I of IPN01195, a RAF inhibitor, complementing IPN01194, an ERK inhibitor, and tovorafenib, two other assets targeting the MAPK pathway.
Group refinancing
Ipsen announced on March 19th the successful completion of its inaugural Rated Public Bond of €500 million with a coupon of 3.875%, maturing in March 2032. Following the disclosure of the Investment Grade ratings from S&P and Moody’s, this transaction was very well received and largely oversubscribed by a diversified and solid institutional investor base. This transaction is an important component of Ipsen’s refinancing plan which included the successful renewal of €1,5 billion syndicated Revolving Credit Facility, extending Ipsen’s debt maturity profile.
Conference call
A conference call and webcast for investors and analysts will begin today at 2pm CET. Participants can access the call and its details by registering here; webcast details can be found here.
Calendar
Ipsen intends to publish its half-year results on July 31st, 2025.
Notes
All financial figures are in € millions (€m). The performance shown covers the three-month period to 31 March 2025 (Q1 2025, the quarter), compared to the three-month period to 31 March 2024 (Q1 2024), unless stated otherwise.
About Ipsen
We are a global biopharmaceutical company with a focus on bringing transformative medicines to patients in three therapeutic areas: Oncology, Rare Disease and Neuroscience.
Our pipeline is fueled by internal and external innovation and supported by nearly 100 years of development experience and global hubs in the U.S., France and the U.K. Our teams in more than 40 countries and our partnerships around the world enable us to bring medicines to patients in more than 100 countries.
Ipsen is listed in Paris (Euronext: IPN) and in the U.S. through a Sponsored Level I American Depositary Receipt program (ADR: IPSEY). For more information, visit ipsen.com.
Ipsen contacts
InvestorsKhalid Deojee +33 6 69 09 12 96
MediaSally Bain +1 857 320 0517Anne Liontas +33 7 67 34 72 96
Disclaimers and/or forward-looking statements
The forward-looking statements, objectives and targets contained herein are based on Ipsen’s management strategy, current views and assumptions. Such statements involve known and unknown risks and uncertainties that may cause actual results, performance or events to differ materially from those anticipated herein. All of the above risks could affect Ipsen’s future ability to achieve its financial targets, which were set assuming reasonable macroeconomic conditions based on the information available today. Use of the words ‘believes’, ‘anticipates’ and ‘expects’ and similar expressions are intended to identify forward-looking statements, including Ipsen’s expectations regarding future events, including regulatory filings and determinations. Moreover, the targets described in this document were prepared without taking into account external-growth assumptions and potential future acquisitions, which may alter these parameters. These objectives are based on data and assumptions regarded as reasonable by Ipsen. These targets depend on conditions or facts likely to happen in the future, and not exclusively on historical data. Actual results may depart significantly from these targets given the occurrence of certain risks and uncertainties, notably the fact that a promising medicine in early development phase or clinical trial may end up never being launched on the market or reaching its commercial targets, notably for regulatory or competition reasons. Ipsen must face or might face competition from generic medicine that might translate into a loss of market share. Furthermore, the research and development process involves several stages each of which involves the substantial risk that Ipsen may fail to achieve its objectives and be forced to abandon its efforts with regards to a medicine in which it has invested significant sums. Therefore, Ipsen cannot be certain that favorable results obtained during preclinical trials will be confirmed subsequently during clinical trials, or that the results of clinical trials will be sufficient to demonstrate the safe and effective nature of the medicine concerned. There can be no guarantees a medicine will receive the necessary regulatory approvals or that the medicine will prove to be commercially successful. If underlying assumptions prove inaccurate or risks or uncertainties materialize, actual results may differ materially from those set forth in the forward-looking statements. Other risks and uncertainties include but are not limited to, general industry conditions and competition; general economic factors, including interest rate and currency exchange rate fluctuations; the impact of pharmaceutical industry regulation and healthcare legislation; global trends toward healthcare cost containment; technological advances, new medicine and patents attained by competitors; challenges inherent in new-medicine development, including obtaining regulatory approval; Ipsen’s ability to accurately predict future market conditions; manufacturing difficulties or delays; financial instability of international economies and sovereign risk; dependence on the effectiveness of Ipsen’s patents and other protections for innovative medicines; and the exposure to litigation, including patent litigation, and/or regulatory actions. Ipsen also depends on third parties to develop and market some of its medicines which could potentially generate substantial royalties; these partners could behave in such ways which could cause damage to Ipsen’s activities and financial results. Ipsen cannot be certain that its partners will fulfil their obligations. It might be unable to obtain any benefit from those agreements. A default by any of Ipsen’s partners could generate lower revenues than expected. Such situations could have a negative impact on Ipsen’s business, financial position or performance. Ipsen expressly disclaims any obligation or undertaking to update or revise any forward-looking statements, targets or estimates contained in this press release to reflect any change in events, conditions, assumptions or circumstances on which any such statements are based, unless so required by applicable law. Ipsen’s business is subject to the risk factors outlined in its registration documents filed with the French Autorité des Marchés Financiers. The risks and uncertainties set out are not exhaustive and the reader is advised to refer to Ipsen’s latest Universal Registration Document, available on ipsen.com.
1 At constant exchange rates (CER), which exclude any foreign-exchange impact by recalculating the performance for the relevant period by applying the exchange rates used for the prior period.2 EMA: European Medicines Agency 3 Long-acting neurotoxin
Attachment
Ipsen PR_Q1 2025_15042025
Total sales growth of 11.6% at CER1, or 11.7% as reported, driven by all three therapeutic areas and including an increasing contribution from Iqirvo and Bylvay. Tovorafenib regulatory submission to EMA2 for pediatric low-grade glioma. Confirmation of full-year 2025 financial guidance.
PARIS, FRANCE, 16 April 2025 – Ipsen (Euronext: IPN; ADR: IPSEY), a global specialty-care biopharmaceutical company, today presents sales for the first quarter of 2025.
“Ipsen has delivered a strong start to 2025, building further momentum in the transformation of our company,” commented David Loew, Chief Executive Officer, Ipsen. “We continued to execute on our strategy with strong top-line growth and pipeline progression. I am pleased to see the rapid build-up of our Rare Cholestatic Liver disease franchise with two innovate medicines for five indications. 2025 is set to be an important year for Ipsen, with multiple launches underway and several milestones expected across our portfolio.”
Full-year guidance
Ipsen is confirming financial guidance for full-year 2025:
Total sales growth greater than 5.0%, at constant currency. Based on the average level of exchange rates in March 2025, a limited effect on total sales from currencies is expected. Core operating margin greater than 30.0% of total sales, which includes additional R&D expenses from anticipated early and mid-stage external-innovation opportunities.
Guidance includes expected negative impact on Somatuline sales due to increased generic competition in the U.S. and Europe. It excludes any impact from potential late-stage (Phase III clinical development or later) business development transactions.
Upcoming Milestones
Ipsen anticipates several key milestones across its portfolio in 2025, including:
Cabometyx (CABINET trial) – Regulatory decision in the European Union for advanced pancreatic (pNETs) and extra-pancreatic (epNETs) neuroendocrine tumors (NETs). fidrisertib (FALKON trial) – Readout of the pivotal Phase IIb trial in fibrodysplasia ossificans progressiva (FOP). LANT3 (LANTIC trial) – Proof-of-concept data readout, evaluating its potential in aesthetics.
Q1 pipeline progress
The regulatory filing for tovorafenib was accepted by EMA for review in the European Union, marking an important step forward in the development of this potential treatment for pediatric low-grade glioma and reinforcing Ipsen’s commitment to innovation in rare and difficult-to-treat cancers.
Ipsen also initiated the entry in Phase I of IPN01195, a RAF inhibitor, complementing IPN01194, an ERK inhibitor, and tovorafenib, two other assets targeting the MAPK pathway.
Group refinancing
Ipsen announced on March 19th the successful completion of its inaugural Rated Public Bond of €500 million with a coupon of 3.875%, maturing in March 2032. Following the disclosure of the Investment Grade ratings from S&P and Moody’s, this transaction was very well received and largely oversubscribed by a diversified and solid institutional investor base. This transaction is an important component of Ipsen’s refinancing plan which included the successful renewal of €1,5 billion syndicated Revolving Credit Facility, extending Ipsen’s debt maturity profile.
Conference call
A conference call and webcast for investors and analysts will begin today at 2pm CET. Participants can access the call and its details by registering here; webcast details can be found here.
Calendar
Ipsen intends to publish its half-year results on July 31st, 2025.
Notes
All financial figures are in € millions (€m). The performance shown covers the three-month period to 31 March 2025 (Q1 2025, the quarter), compared to the three-month period to 31 March 2024 (Q1 2024), unless stated otherwise.
About Ipsen
We are a global biopharmaceutical company with a focus on bringing transformative medicines to patients in three therapeutic areas: Oncology, Rare Disease and Neuroscience.
Our pipeline is fueled by internal and external innovation and supported by nearly 100 years of development experience and global hubs in the U.S., France and the U.K. Our teams in more than 40 countries and our partnerships around the world enable us to bring medicines to patients in more than 100 countries.
Ipsen is listed in Paris (Euronext: IPN) and in the U.S. through a Sponsored Level I American Depositary Receipt program (ADR: IPSEY). For more information, visit ipsen.com.
Ipsen contacts
InvestorsKhalid Deojee +33 6 69 09 12 96
MediaSally Bain +1 857 320 0517Anne Liontas +33 7 67 34 72 96
Disclaimers and/or forward-looking statements
The forward-looking statements, objectives and targets contained herein are based on Ipsen’s management strategy, current views and assumptions. Such statements involve known and unknown risks and uncertainties that may cause actual results, performance or events to differ materially from those anticipated herein. All of the above risks could affect Ipsen’s future ability to achieve its financial targets, which were set assuming reasonable macroeconomic conditions based on the information available today. Use of the words ‘believes’, ‘anticipates’ and ‘expects’ and similar expressions are intended to identify forward-looking statements, including Ipsen’s expectations regarding future events, including regulatory filings and determinations. Moreover, the targets described in this document were prepared without taking into account external-growth assumptions and potential future acquisitions, which may alter these parameters. These objectives are based on data and assumptions regarded as reasonable by Ipsen. These targets depend on conditions or facts likely to happen in the future, and not exclusively on historical data. Actual results may depart significantly from these targets given the occurrence of certain risks and uncertainties, notably the fact that a promising medicine in early development phase or clinical trial may end up never being launched on the market or reaching its commercial targets, notably for regulatory or competition reasons. Ipsen must face or might face competition from generic medicine that might translate into a loss of market share. Furthermore, the research and development process involves several stages each of which involves the substantial risk that Ipsen may fail to achieve its objectives and be forced to abandon its efforts with regards to a medicine in which it has invested significant sums. Therefore, Ipsen cannot be certain that favorable results obtained during preclinical trials will be confirmed subsequently during clinical trials, or that the results of clinical trials will be sufficient to demonstrate the safe and effective nature of the medicine concerned. There can be no guarantees a medicine will receive the necessary regulatory approvals or that the medicine will prove to be commercially successful. If underlying assumptions prove inaccurate or risks or uncertainties materialize, actual results may differ materially from those set forth in the forward-looking statements. Other risks and uncertainties include but are not limited to, general industry conditions and competition; general economic factors, including interest rate and currency exchange rate fluctuations; the impact of pharmaceutical industry regulation and healthcare legislation; global trends toward healthcare cost containment; technological advances, new medicine and patents attained by competitors; challenges inherent in new-medicine development, including obtaining regulatory approval; Ipsen’s ability to accurately predict future market conditions; manufacturing difficulties or delays; financial instability of international economies and sovereign risk; dependence on the effectiveness of Ipsen’s patents and other protections for innovative medicines; and the exposure to litigation, including patent litigation, and/or regulatory actions. Ipsen also depends on third parties to develop and market some of its medicines which could potentially generate substantial royalties; these partners could behave in such ways which could cause damage to Ipsen’s activities and financial results. Ipsen cannot be certain that its partners will fulfil their obligations. It might be unable to obtain any benefit from those agreements. A default by any of Ipsen’s partners could generate lower revenues than expected. Such situations could have a negative impact on Ipsen’s business, financial position or performance. Ipsen expressly disclaims any obligation or undertaking to update or revise any forward-looking statements, targets or estimates contained in this press release to reflect any change in events, conditions, assumptions or circumstances on which any such statements are based, unless so required by applicable law. Ipsen’s business is subject to the risk factors outlined in its registration documents filed with the French Autorité des Marchés Financiers. The risks and uncertainties set out are not exhaustive and the reader is advised to refer to Ipsen’s latest Universal Registration Document, available on ipsen.com.
1 At constant exchange rates (CER), which exclude any foreign-exchange impact by recalculating the performance for the relevant period by applying the exchange rates used for the prior period.2 EMA: European Medicines Agency 3 Long-acting neurotoxin
Attachment
Ipsen PR_Q1 2025_15042025
Phase 2Phase 1Financial Statement
13 Feb 2025
FY 2024 total sales growth of 9.9% at CER1, or 8.7% as reported, with growth driven by strong performance across all therapeutic areas, including a 67.4% increase in the Rare Diseases portfolio, 9.2% in Neuroscience, and 7.3% in Oncology; Somatuline® (lanreotide) sales grew by 5.6%, while all other products, excluding Somatuline, achieved double-digit sales growth at 12.2% FY 2024 core operating income of €1,109m, growing 10.8% as reported, with core operating margin of 32.6% of total sales Continued pipeline expansion in 2024, with significant regulatory approvals, addition of several preclinical therapies with global rights and innovative modalities, and a late-stage asset Four key regulatory and clinical milestones expected in 2025, including the Proof-of-Concept data readout for the Long-Acting Neurotoxin (LANT) Financial guidance2 for 2025 including total sales growth greater than 5.0%3 at CER, and core operating margin greater than 30.0% of total sales, based on accelerated sales growth of the ex-Somatuline portfolio and assuming negative impact on Somatuline sales due to increased generic competition in the U.S. and Europe
PARIS, FRANCE, 13 February 2025 – Ipsen (Euronext: IPN; ADR: IPSEY), a global specialty-care biopharmaceutical company, today presents its financial results for the full year 2024.
“Ipsen delivered solid results and advanced its pipeline in 2024, laying a strong foundation for sustained growth,” said David Loew, Chief Executive Officer, Ipsen. “With the successful global rollout of Iqirvo and Bylvay, and the U.S. launch of Onivyde, alongside multiple business development deals adding several innovative assets, we are well positioned to execute our strategic roadmap. This year, we look forward to achieving key milestones, including the first data readout for the Long-Acting Neurotoxin (LANT), and further expand and progress our pipeline across all three therapeutic areas to bring promising new medicines to patients.”
Pipeline Progress
Significant regulatory milestones were achieved in 2024, including FDA approval of Onivyde® (irinotecan) for first-line pancreatic ductal adenocarcinoma (PDAC), along with accelerated U.S. approval and European approval for Iqirvo® (elafibranor), respectively. Additionally, Kayfanda® (odevixibat) was approved for Alagille syndrome (ALGS) in the E.U.
The company also opted-in for the CABINET Phase III study of Cabometyx® (cabozantinib) in patients with advanced neuroendocrine tumors (NETs), with study results presented at the 2024 European Society for Medical Oncology (ESMO) Congress and published in the New England Journal of Medicine.
An IND application was filed for IPN01194, an ERK inhibitor, advancing the potential medicine into clinical development with a Phase I/IIa trial in advanced solid tumors.
Ipsen improved further the depth and breadth of its pipeline by adding five preclinical innovative therapies with global rights and new modalities, and an ex-U.S. licensing agreement with DayOne Biopharmaceuticals for the late-stage oncology asset tovorafenib, an oral RAF inhibitor for pediatric low-grade glioma.
Two global licensing agreements for Antibody Drug Conjugate (ADC) in oncology with Sutro Biopharma and Foreseen Biotechnology were signed. An extension of the oncology partnership with Marengo Therapeutics to include TriSTAR, a next-generation precision T-cell engager was completed, as well as more recently, in the fourth quarter, a global licensing agreement with Biomunex for a preclinical novel T-cell engager (TCE). A collaboration with Skyhawk Therapeutics to develop RNA-modulating small molecules for rare neurological diseases was also signed during the year.
Ipsen executed several divestments in 2024, including the sale of Increlex® (mecasermin injection) to Eton Pharmaceuticals and the sale of its rare pediatric disease Priority Review Voucher.
Environmental, Social and Governance
Ipsen took important steps in 2024 in delivering its ambitious sustainability strategy. The company continued to integrate sustainability across its operations. From reducing its environmental footprint to advancing patient access and fostering a strong workplace culture, the company increased its commitment to driving progress for patients, employees, communities, and the planet.
Our sustainability efforts were recognized across multiple environment initiatives. The company achieved a 45% reduction in Scopes 1 & 2 greenhouse gas emissions and a 25% reduction in Scope 3, fully in line with its 2030 targets (versus 2019 baseline). Significant efforts were made to engage suppliers and third parties in Ipsen’s sustainability roadmap including the first-ever “Ipsen Supplier Sustainability Day”. Following an intensive transformation project, 99.8% of Ipsen’s global electricity now comes from renewable sources. Through the Fleet for Future project, the company continues to advance sustainable transportation, with 43% of its total company’s fleet now electric vehicles as of 2024.
We remain committed to gender balance in leadership, with women now representing 55% of the Global Leadership Team.
2025 Upcoming Milestones
Ipsen anticipates several key milestones across its portfolio in 2025, including:
Cabometyx (CABINET trial) – Regulatory decision in Europe for advanced neuroendocrine tumors (NETs), including pancreatic (pNETs) and extra-pancreatic (epNETs) neuroendocrine tumors Tovorafenib (FIREFLY-1 trial) – Regulatory submission in Europe for pediatric low-grade glioma Fidrisertib (FALKON trial) – Readout of the pivotal Phase IIb trial in fibrodysplasia ossificans progressiva (FOP) LANT88(LANTIC trial) – Proof-of-concept data readout, evaluating its potential in aesthetics
These milestones reinforce Ipsen’s commitment to advancing innovative therapies and expanding treatment options for patients worldwide.
2025 Financial Guidance
Ipsen has set for FY 2025 the following financial guidance, which excludes any impact from potential late-stage (Phase III clinical development or later) business development transactions:
Total sales growth greater than 5.0%, at constant currency. Based on the average level of exchange rates in January 2025, a favorable effect on total sales of around 1% from currencies is expected. Core operating margin greater than 30.0% of total sales, which includes additional R&D expenses from anticipated early and mid-stage external-innovation opportunities.
Guidance on total sales and core operating margin is based on accelerated sales growth of the ex-Somatuline portfolio and assumes negative impact on Somatuline sales due to increased generic competition in the U.S. and Europe.
Consolidated financial statements
The Board of Directors approved the consolidated financial statements on 12 February 2025. The consolidated financial statements have been audited and the Statutory Auditors’ report is in the process of being published. Ipsen’s comprehensive audited financial statements will be available in due course on ipsen.com (regulated-information section).
Conference call
A conference call and webcast for investors and analysts will begin today at 1pm CET. Participants can access the call and its details by registering here; webcast details can be found here.
Calendar
Ipsen intends to publish its Q1 2025 sales on April 16th, 2025.
Notes
All financial figures are in € millions (€m), unless otherwise noted. The performance shown in this announcement covers the twelve-month period to 31 December 2024 (FY 2024) and the three-month period to 31 December 2024 (Q4 2024), compared to the twelve-month period to 31 December 2023 (FY 2023) and the three-month period to 31 December 2023 (Q4 2023), respectively, unless stated otherwise. The commentary is based on the performance in FY 2024, unless stated otherwise.
About Ipsen
We are a global biopharmaceutical company with a focus on bringing transformative medicines to patients in three therapeutic areas: Oncology, Rare Disease and Neuroscience.
Our pipeline is fueled by external innovation and supported by nearly 100 years of development experience and global hubs in the U.S., France and the U.K. Our teams in more than 40 countries and our partnerships around the world enable us to bring medicines to patients in more than 100 countries.
Ipsen is listed in Paris (Euronext: IPN) and in the U.S. through a Sponsored Level I American Depositary Receipt program (ADR: IPSEY). For more information, visit ipsen.com.
Ipsen contacts
Investors
Media
1 At constant exchange rates (CER), which exclude any foreign-exchange impact by recalculating the performance for the relevant period by applying the exchange rates used for the prior period.2 Excluding any impact from potential late-stage (Phase III clinical development or later) external-innovation transactions.3 Based on the average level of exchange rates in Jan 2024, a favorable effect on total sales of about 1% from currencies is expected. 4 Extract of consolidated results. The Company’s auditors performed a limited review of the condensed consolidated financial statements.5 Including an impairment loss of €279m (or €2,33 /share) related to Sohonos, reflecting lower revised sales following lower patient uptake.6 Dividend related to the current financial year to be paid the following year.7 Decided by the Ipsen S.A. Board of Directors and to be proposed at the annual shareholders’ meeting on 21 May 2025.
8 Long-acting neurotoxin
Attachment
Ipsen PR_FY 2024 – Results announcement_13022025
FY 2024 total sales growth of 9.9% at CER1, or 8.7% as reported, with growth driven by strong performance across all therapeutic areas, including a 67.4% increase in the Rare Diseases portfolio, 9.2% in Neuroscience, and 7.3% in Oncology; Somatuline® (lanreotide) sales grew by 5.6%, while all other products, excluding Somatuline, achieved double-digit sales growth at 12.2% FY 2024 core operating income of €1,109m, growing 10.8% as reported, with core operating margin of 32.6% of total sales Continued pipeline expansion in 2024, with significant regulatory approvals, addition of several preclinical therapies with global rights and innovative modalities, and a late-stage asset Four key regulatory and clinical milestones expected in 2025, including the Proof-of-Concept data readout for the Long-Acting Neurotoxin (LANT) Financial guidance2 for 2025 including total sales growth greater than 5.0%3 at CER, and core operating margin greater than 30.0% of total sales, based on accelerated sales growth of the ex-Somatuline portfolio and assuming negative impact on Somatuline sales due to increased generic competition in the U.S. and Europe
PARIS, FRANCE, 13 February 2025 – Ipsen (Euronext: IPN; ADR: IPSEY), a global specialty-care biopharmaceutical company, today presents its financial results for the full year 2024.
“Ipsen delivered solid results and advanced its pipeline in 2024, laying a strong foundation for sustained growth,” said David Loew, Chief Executive Officer, Ipsen. “With the successful global rollout of Iqirvo and Bylvay, and the U.S. launch of Onivyde, alongside multiple business development deals adding several innovative assets, we are well positioned to execute our strategic roadmap. This year, we look forward to achieving key milestones, including the first data readout for the Long-Acting Neurotoxin (LANT), and further expand and progress our pipeline across all three therapeutic areas to bring promising new medicines to patients.”
Pipeline Progress
Significant regulatory milestones were achieved in 2024, including FDA approval of Onivyde® (irinotecan) for first-line pancreatic ductal adenocarcinoma (PDAC), along with accelerated U.S. approval and European approval for Iqirvo® (elafibranor), respectively. Additionally, Kayfanda® (odevixibat) was approved for Alagille syndrome (ALGS) in the E.U.
The company also opted-in for the CABINET Phase III study of Cabometyx® (cabozantinib) in patients with advanced neuroendocrine tumors (NETs), with study results presented at the 2024 European Society for Medical Oncology (ESMO) Congress and published in the New England Journal of Medicine.
An IND application was filed for IPN01194, an ERK inhibitor, advancing the potential medicine into clinical development with a Phase I/IIa trial in advanced solid tumors.
Ipsen improved further the depth and breadth of its pipeline by adding five preclinical innovative therapies with global rights and new modalities, and an ex-U.S. licensing agreement with DayOne Biopharmaceuticals for the late-stage oncology asset tovorafenib, an oral RAF inhibitor for pediatric low-grade glioma.
Two global licensing agreements for Antibody Drug Conjugate (ADC) in oncology with Sutro Biopharma and Foreseen Biotechnology were signed. An extension of the oncology partnership with Marengo Therapeutics to include TriSTAR, a next-generation precision T-cell engager was completed, as well as more recently, in the fourth quarter, a global licensing agreement with Biomunex for a preclinical novel T-cell engager (TCE). A collaboration with Skyhawk Therapeutics to develop RNA-modulating small molecules for rare neurological diseases was also signed during the year.
Ipsen executed several divestments in 2024, including the sale of Increlex® (mecasermin injection) to Eton Pharmaceuticals and the sale of its rare pediatric disease Priority Review Voucher.
Environmental, Social and Governance
Ipsen took important steps in 2024 in delivering its ambitious sustainability strategy. The company continued to integrate sustainability across its operations. From reducing its environmental footprint to advancing patient access and fostering a strong workplace culture, the company increased its commitment to driving progress for patients, employees, communities, and the planet.
Our sustainability efforts were recognized across multiple environment initiatives. The company achieved a 45% reduction in Scopes 1 & 2 greenhouse gas emissions and a 25% reduction in Scope 3, fully in line with its 2030 targets (versus 2019 baseline). Significant efforts were made to engage suppliers and third parties in Ipsen’s sustainability roadmap including the first-ever “Ipsen Supplier Sustainability Day”. Following an intensive transformation project, 99.8% of Ipsen’s global electricity now comes from renewable sources. Through the Fleet for Future project, the company continues to advance sustainable transportation, with 43% of its total company’s fleet now electric vehicles as of 2024.
We remain committed to gender balance in leadership, with women now representing 55% of the Global Leadership Team.
2025 Upcoming Milestones
Ipsen anticipates several key milestones across its portfolio in 2025, including:
Cabometyx (CABINET trial) – Regulatory decision in Europe for advanced neuroendocrine tumors (NETs), including pancreatic (pNETs) and extra-pancreatic (epNETs) neuroendocrine tumors Tovorafenib (FIREFLY-1 trial) – Regulatory submission in Europe for pediatric low-grade glioma Fidrisertib (FALKON trial) – Readout of the pivotal Phase IIb trial in fibrodysplasia ossificans progressiva (FOP) LANT88(LANTIC trial) – Proof-of-concept data readout, evaluating its potential in aesthetics
These milestones reinforce Ipsen’s commitment to advancing innovative therapies and expanding treatment options for patients worldwide.
2025 Financial Guidance
Ipsen has set for FY 2025 the following financial guidance, which excludes any impact from potential late-stage (Phase III clinical development or later) business development transactions:
Total sales growth greater than 5.0%, at constant currency. Based on the average level of exchange rates in January 2025, a favorable effect on total sales of around 1% from currencies is expected. Core operating margin greater than 30.0% of total sales, which includes additional R&D expenses from anticipated early and mid-stage external-innovation opportunities.
Guidance on total sales and core operating margin is based on accelerated sales growth of the ex-Somatuline portfolio and assumes negative impact on Somatuline sales due to increased generic competition in the U.S. and Europe.
Consolidated financial statements
The Board of Directors approved the consolidated financial statements on 12 February 2025. The consolidated financial statements have been audited and the Statutory Auditors’ report is in the process of being published. Ipsen’s comprehensive audited financial statements will be available in due course on ipsen.com (regulated-information section).
Conference call
A conference call and webcast for investors and analysts will begin today at 1pm CET. Participants can access the call and its details by registering here; webcast details can be found here.
Calendar
Ipsen intends to publish its Q1 2025 sales on April 16th, 2025.
Notes
All financial figures are in € millions (€m), unless otherwise noted. The performance shown in this announcement covers the twelve-month period to 31 December 2024 (FY 2024) and the three-month period to 31 December 2024 (Q4 2024), compared to the twelve-month period to 31 December 2023 (FY 2023) and the three-month period to 31 December 2023 (Q4 2023), respectively, unless stated otherwise. The commentary is based on the performance in FY 2024, unless stated otherwise.
About Ipsen
We are a global biopharmaceutical company with a focus on bringing transformative medicines to patients in three therapeutic areas: Oncology, Rare Disease and Neuroscience.
Our pipeline is fueled by external innovation and supported by nearly 100 years of development experience and global hubs in the U.S., France and the U.K. Our teams in more than 40 countries and our partnerships around the world enable us to bring medicines to patients in more than 100 countries.
Ipsen is listed in Paris (Euronext: IPN) and in the U.S. through a Sponsored Level I American Depositary Receipt program (ADR: IPSEY). For more information, visit ipsen.com.
Ipsen contacts
Investors
Media
1 At constant exchange rates (CER), which exclude any foreign-exchange impact by recalculating the performance for the relevant period by applying the exchange rates used for the prior period.2 Excluding any impact from potential late-stage (Phase III clinical development or later) external-innovation transactions.3 Based on the average level of exchange rates in Jan 2024, a favorable effect on total sales of about 1% from currencies is expected. 4 Extract of consolidated results. The Company’s auditors performed a limited review of the condensed consolidated financial statements.5 Including an impairment loss of €279m (or €2,33 /share) related to Sohonos, reflecting lower revised sales following lower patient uptake.6 Dividend related to the current financial year to be paid the following year.7 Decided by the Ipsen S.A. Board of Directors and to be proposed at the annual shareholders’ meeting on 21 May 2025.
8 Long-acting neurotoxin
Attachment
Ipsen PR_FY 2024 – Results announcement_13022025
Phase 2Drug ApprovalLicense out/inPhase 3
15 Jul 2023
DUBLIN, July 14, 2023 /PRNewswire/ -- The "Fibrodysplasia Ossificans Progressiva - Pipeline Insight, 2023" clinical trials has been added to
ResearchAndMarkets.com's offering.
With a focus on 5+ companies and 7+ pipeline drugs, this report provides a detailed analysis of the advancements and potential breakthroughs in Fibrodysplasia ossificans progressiva treatment.
The report dives into pipeline drug profiles, encompassing both clinical and nonclinical stage products. It also includes a thorough assessment of therapeutics based on product type, stage, route of administration, and molecule type. In addition, the report sheds light on the inactive pipeline products within this space, providing a comprehensive overview of the Fibrodysplasia ossificans progressiva pipeline landscape.
Within the field of Fibrodysplasia ossificans progressiva research and development, companies and academics are diligently working to assess challenges and seek opportunities. The therapies currently under development focus on novel approaches to treat and improve Fibrodysplasia ossificans progressiva. This report unveils these innovative therapies and offers insights into the potential future of Fibrodysplasia ossificans progressiva treatment.
Stay informed and gain a competitive edge with this comprehensive guide to the latest advancements and pipeline drugs in the field of Fibrodysplasia ossificans progressiva treatment.
Fibrodysplasia ossificans progressiva Emerging Drugs Chapters
This segment of the Fibrodysplasia ossificans progressiva report encloses its detailed analysis of various drugs in different stages of clinical development, including phase II, I, preclinical and Discovery. It also helps to understand clinical trial details, expressive pharmacological action, agreements and collaborations, and the latest news and press releases.
Fibrodysplasia ossificans progressiva Emerging Drugs
REGN2477: Regeneron Pharmaceuticals
REGN2477 (also known as garetosmab) is an antibody that binds to Activin A and blocks its activity. By binding and blocking Activin A, REN2477 prevents the formation and growth of HO in FOP. REGN2477 has received Orphan Drug status for FOP from the US Food and Drug Administration (FDA), and orphan status for the treatment of FOP in the EU. Currently, the drug is being evaluated in the Phase III clinical trial for the treatment of Fibrodysplasia ossificans progressiva.
IPN60130: Ipsen
IPN60130 (Fidrisertib) is an oral investigational drug designed to selectively target the mutant FOP receptor (ACVR1/ALK2), the underlying cause of FOP. FDA has granted Fast Track Designation to IPN60130 for the treatment of FOP. Currently, the drug is being evaluated in the Phase II clinical trial for the treatment of Fibrodysplasia ossificans progressiva.
Fibrodysplasia ossificans progressiva: Therapeutic Assessment
This segment of the report provides insights about the different Fibrodysplasia ossificans progressiva drugs segregated based on following parameters that define the scope of the report, such as:
Major Players in Fibrodysplasia ossificans progressiva
There are approx. 5+ key companies which are developing the therapies for Fibrodysplasia ossificans progressiva. The companies which have their Fibrodysplasia ossificans progressiva drug candidates in the most advanced stage, i.e. phase III include, Regeneron Pharmaceuticals.
Phases
The report covers around 7+ products under different phases of clinical development like
Late stage products (Phase III)
Mid-stage products (Phase II)
Early-stage product (Phase I) along with the details of
Pre-clinical and Discovery stage candidates
Discontinued & Inactive candidates
Route of Administration
Fibrodysplasia ossificans progressiva Report Insights
Fibrodysplasia ossificans progressiva Pipeline Analysis
Therapeutic Assessment
Unmet Needs
Impact of Drugs
Fibrodysplasia ossificans progressiva Report Assessment
Pipeline Product Profiles
Therapeutic Assessment
Pipeline Assessment
Inactive drugs assessment
Unmet Needs
Key Players
Regeneron Pharmaceuticals
Ipsen
Incyte Corporation
Daiichi Sankyo
Key Products
REGN2477
IPN60130
DS-6016a
INCB000928
For more information about this clinical trials report visit
About ResearchAndMarkets.com
ResearchAndMarkets.com is the world's leading source for international market research reports and market data. We provide you with the latest data on international and regional markets, key industries, the top companies, new products and the latest trends.
Media Contact:
Research and Markets
Laura Wood, Senior Manager
[email protected]
For E.S.T Office Hours Call +1-917-300-0470
For U.S./CAN Toll Free Call +1-800-526-8630
For GMT Office Hours Call +353-1-416-8900
U.S. Fax: 646-607-1907
Fax (outside U.S.): +353-1-481-1716
Logo:
SOURCE Research and Markets
Orphan DrugFast TrackPhase 2Phase 3
100 Deals associated with Fidrisertib succinate
Login to view more data
R&D Status
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| Myositis Ossificans | Phase 2 | United States | 01 Dec 2021 | |
| Myositis Ossificans | Phase 2 | China | 01 Dec 2021 | |
| Myositis Ossificans | Phase 2 | Japan | 01 Dec 2021 | |
| Myositis Ossificans | Phase 2 | Argentina | 01 Dec 2021 | |
| Myositis Ossificans | Phase 2 | Australia | 01 Dec 2021 | |
| Myositis Ossificans | Phase 2 | Belgium | 01 Dec 2021 | |
| Myositis Ossificans | Phase 2 | Canada | 01 Dec 2021 | |
| Myositis Ossificans | Phase 2 | France | 01 Dec 2021 | |
| Myositis Ossificans | Phase 2 | Italy | 01 Dec 2021 | |
| Myositis Ossificans | Phase 2 | Mexico | 01 Dec 2021 |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
No Data | |||||||
Login to view more data
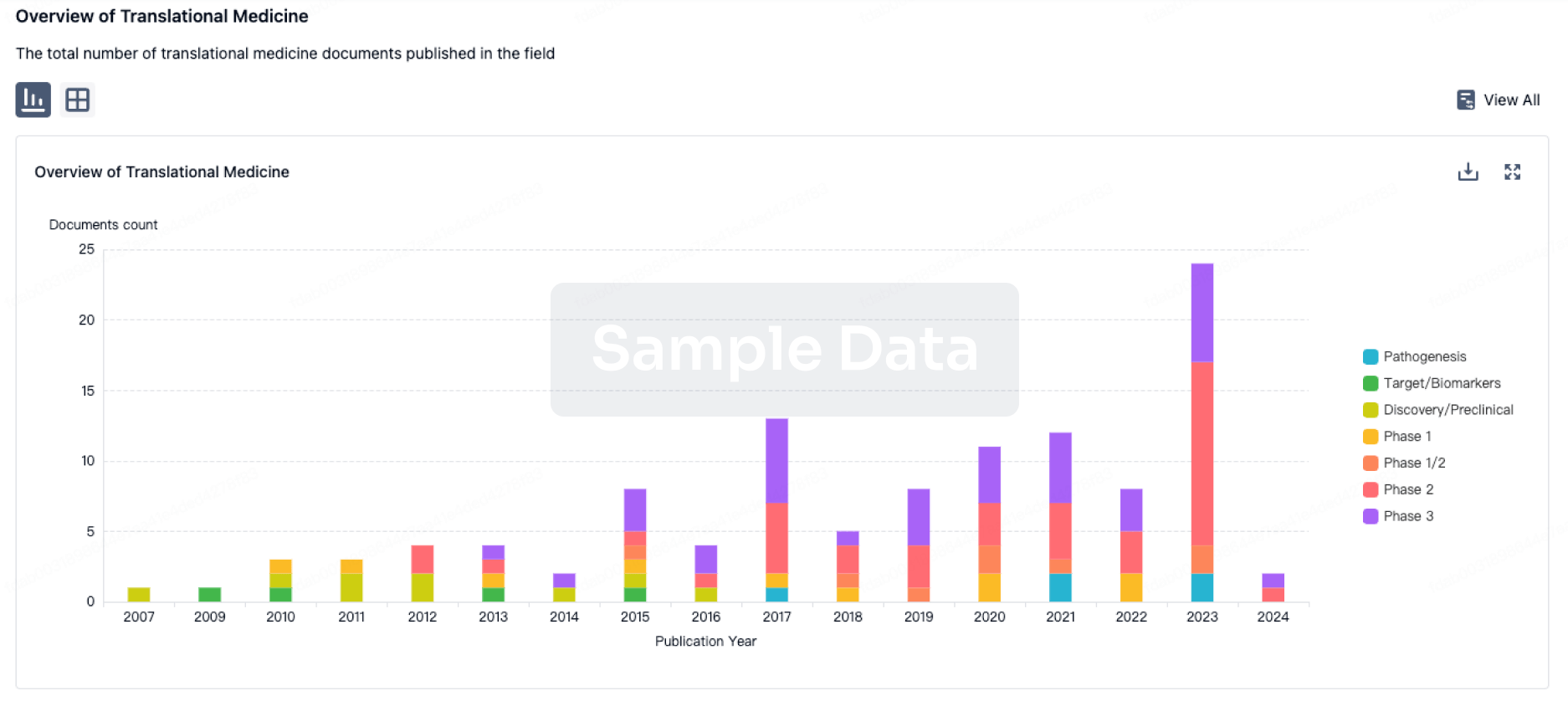
Translational Medicine
Boost your research with our translational medicine data.
login
or
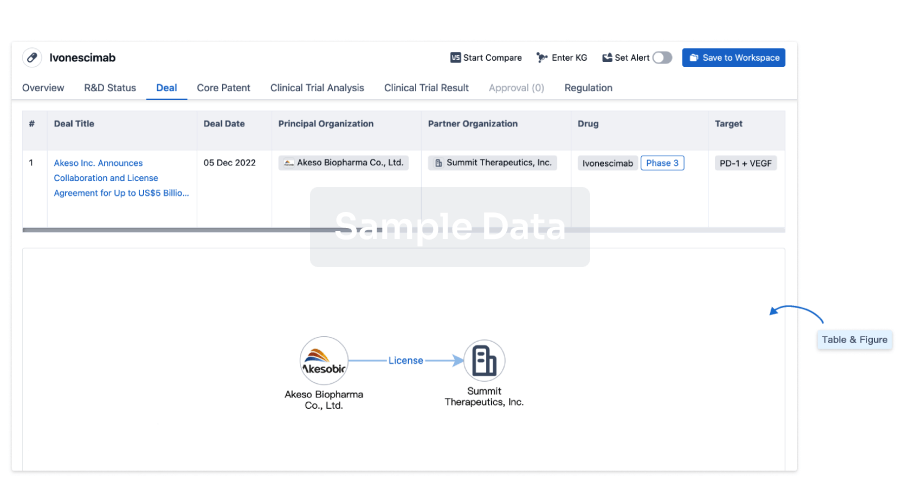
Deal
Boost your decision using our deal data.
login
or
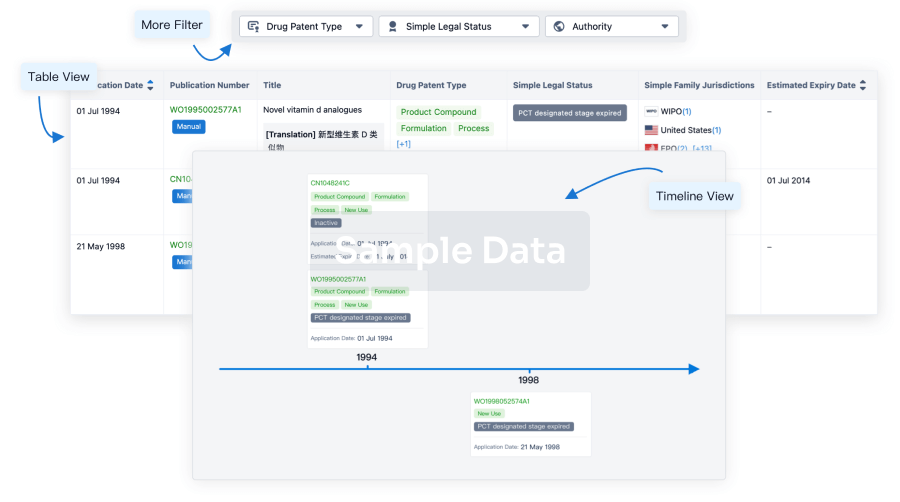
Core Patent
Boost your research with our Core Patent data.
login
or
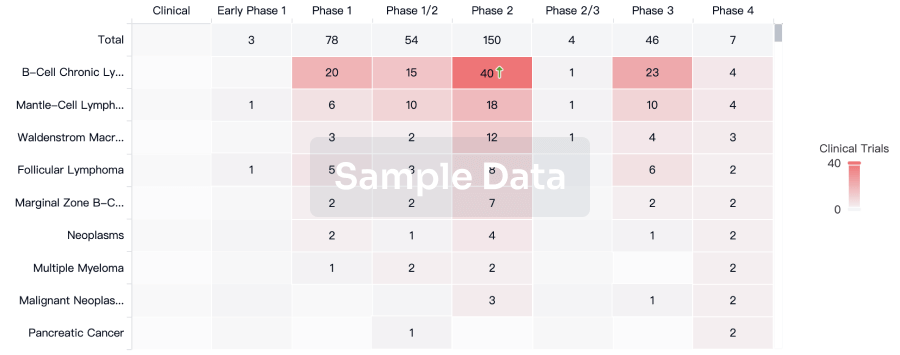
Clinical Trial
Identify the latest clinical trials across global registries.
login
or
Approval
Accelerate your research with the latest regulatory approval information.
login
or

Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or
AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free


