UroMems Initiates First-in-Human Study of Its Smart Implant to Treat Stress Urinary Incontinence
29 Nov 2022
Clinical Study
This initial clinical trial is a key milestone in the development of its UroActive™ System
GRENOBLE, France, Nov. 29, 2022 /PRNewswire/ -- UroMems, a global company developing breakthrough, mechatronics technology to treat stress urinary incontinence (SUI), announced today that it has successfully completed the first-in-human implant of the UroActive™ System, the first smart automated artificial urinary sphincter (AUS) investigational device to treat SUI. This initial clinical study is a key milestone in the development of UroActive.
Continue Reading
Preview
Source: PRNewswire
UroMems, a global company developing breakthrough, mechatronics technology to treat stress urinary incontinence (SUI), announced today that it has successfully completed the first-in-human implant of the UroActive™ System, the first smart automated artificial urinary sphincter (AUS) investigational device to treat SUI. This initial clinical study is a key milestone in the development of UroActive.
"This is a historic milestone for UroMems, patients, physicians and the industry as this is a first-of-its-kind fully automated AUS implant designed to treat SUI in both men and women," said Hamid Lamraoui, UroMems chief executive officer and co-founder. "This is the next important step in delivering our novel technology to a large, underserved patient population with unmet needs not addressed by current options on the market."
The first male patient procedure was successfully completed at La Pitié-Salpêtrière University Hospital (AP-HP, Sorbonne University, Paris, France) by Professor Emmanuel Chartier-Kastler, principal investigator, with approval from the National Agency for the Safety of Medicines and Health Products (or ANSM, the French equivalent to the U.S. Food and Drug Administration).
"We believe this groundbreaking therapy will change the way SUI is treated worldwide, establishing a new standard of care," said Professor Pierre Mozer, UroMems chief medical officer and co-founder.
UroMems aims to restore the quality of life, dignity and self-esteem of millions of men and women worldwide suffering from untreated chronic conditions by the commitment to change the perception that these disorders are inevitable as one grows older and is simply something to endure with no real solution. UroMems is revolutionizing the treatment of SUI with smart active implants, using the latest technological advances in the field of embedded systems and micro-technologies for the development of its groundbreaking solutions.
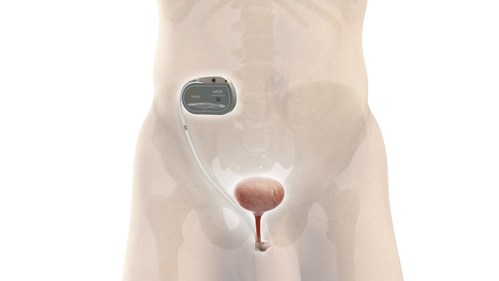
UroActive is the first smart active implant that treats SUI, powered by a MyoElectroMechanical System (MEMS). This innovative system is placed around the urethral duct and is automatically controlled based on the patient's activity, without the need for manual adjustments, intending to provide patients with ease of use and a better quality of life than current options.
"We see a high rate of today's AUS implants that are revised or removed after three years," said Professor Daniel Elliott, urologist at Mayo Clinic in Rochester, Minn., and clinical advisor to UroMems. "The potential of being able to personalize the therapy to the patient automatically, post-implantation is very appealing."
SUI, or involuntary urinary leakage, affects an estimated 40 million Americans and 90 million Europeans, and occurs when the pressure in the bladder exceeds that of the muscle (the sphincter) around the urethra, caused by activities involving high intra-abdominal pressure, like coughing, laughing and exercising. SUI significantly impacts quality of life, as it can be debilitating, and often leads to depression, low self-esteem and social stigma. While mild SUI is addressed by pelvic floor re-education and bulking agents, moderate and severe SUI historically have only had two options: mesh sling or artificial urinary sphincter.
About UroActive
UroActive is an active implantable electronic artificial urinary sphincter that has been developed to compensate for sphincter insufficiency in patients, both men and women, with SUI. It is based on a unique bioelectronic platform using embedded smart, AI-based, digital and robotic systems which, based on data collected from a patient, create a treatment algorithm that is specific for each patient's needs. The UroMems technology platform is protected by more than 100 patents and is designed to overcome the limitations of current solutions by optimizing safety and performance, patient experience and surgeon convenience.
About UroMems
Founded in 2011 by Professor Pierre Mozer, Hamid Lamraoui and Stéphane Lavallée, UroMems aims to restore the quality of life, dignity and self-esteem of millions of men and women worldwide suffering from untreated chronic conditions by the commitment to change the perception that these disorders are inevitable as one grows older and is simply something to endure with no real solution. The first challenge for the company will be applying embedded mechatronics methods and smart systems for treating urinary incontinence: designed by urologists and collaborating scientists and engineers, UroActive intends to provide a new standard of care combining safety, efficacy, durability and ergonomics fitting any individual's lifestyle and anatomy.
Since the inception of the company, significant investments have been made for the development of UroMems' first product. This includes two financing rounds totaling 46 million euros, led by Wellington Partners, Bpifrance, Supernova Invest, b-to-v Partners AG, Cita Investissement, Hil-Invent, Financière Arbevel and the founders. The company has received several awards for innovation, including the Prix Galien Award Medstart'up and the Worldwide Innovation Challenge initiated by the French government. For more information, please visit www.uromems.com.
Media Contact
Shelli Lissick
[email protected]
651-276-6922
SOURCE UroMems
For more details,please visit the original website
The content of the article does not represent any opinions of Synapse and its affiliated companies. If there is any copyright infringement or error, please contact us, and we will deal with it within 24 hours.
Organizations
Targets
-Drugs
-Chat with Hiro
Hot reports
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.





