HUTCHMED Highlights Clinical Data to be Presented at 2023 ESMO Asia and ESMO Immuno-Oncology Congresses
01 Dec 2023
Clinical ResultImmunotherapy
HUTCHMED (China) Limited (“HUTCHMED”) (Nasdaq/AIM:HCM; HKEX:13) today highlights that new clinical data from several ongoing studies with HUTCHMED investigational drug candidates fruquintinib, surufatinib and HMPL-295, which will be presented at the upcoming European Society for Medical Oncology (“ESMO”) Asia Congress, taking place on December 1-3, 2023 in Singapore, and the ESMO Immuno-Oncology Congress, taking place on December 6-8, 2023 in Geneva, Switzerland.
HMPL-295:

Preview
Source: GlobeNewswire-Health Care
This presentation will report data from a multi-center, open-label clinical trial to evaluate safety, tolerability, pharmacokinetics and preliminary efficacy profile of HMPL-295, and to determine the maximum tolerated dose (“MTD”) and recommended Phase II dose in patients with advanced malignant solid tumors. The continuous-administration MTD was determined to be 50 mg QD, and intermittent administration studies are ongoing.
HMPL-295 is an investigational, selective, oral inhibitor of extracellular signal-regulated kinase 1 & 2 (ERK1/2), which is a downstream component of the RAS-MAPK pathway signaling cascade. The investigational compound has the potential to address intrinsic or acquired resistance from upstream mechanisms such as RAS, RAF and MEK. HMPL-295 is one of several investigational compounds discovered by HUTCHMED that target the RAS-MAPK pathway.
Fruquintinib:

Preview
Source: GlobeNewswire-Health Care

Preview
Source: GlobeNewswire-Health Care
These presentations will report results from the cervical cancer and NSCLC patient cohorts of the basket clinical trial in China of fruquintinib plus sintilimab. This trial is an open-label, multi-center, non-randomized, Phase ІІ study to assess the safety and efficacy of fruquintinib in combination with sintilimab in patients with advanced cervical cancer, endometrial cancer (“EMC”), gastric cancer (GC), hepatocellular carcinoma (HCC), NSCLC or renal cell carcinoma (“RCC”). Data from the EMC and RCC cohorts of this trial led to the initiation of registration enabling programs. This combination treatment showed promising antitumor activity in advanced cervical cancer and NSCLC patients, particularly for patients with PD-L1 positive status. This combination treatment also showed manageable toxicity profiles consistent with that seen in other cohorts.
Fruquintinib is a selective oral inhibitor of vascular endothelial growth factor receptors (“VEGFR”) -1, -2 and -3. VEGFR inhibitors play a pivotal role in blocking tumor angiogenesis. Fruquintinib was designed to have enhanced selectivity that limits off-target kinase activity, allowing for high drug exposure, sustained target inhibition, and flexibility for the potential use as part of combination therapy. Fruquintinib has demonstrated a manageable safety profile and is being investigated in combination with other anti-cancer therapies including the approved PD-1 inhibitor, sintilimab.

Preview
Source: GlobeNewswire-Health Care
Investigator-initiated studies presentations:
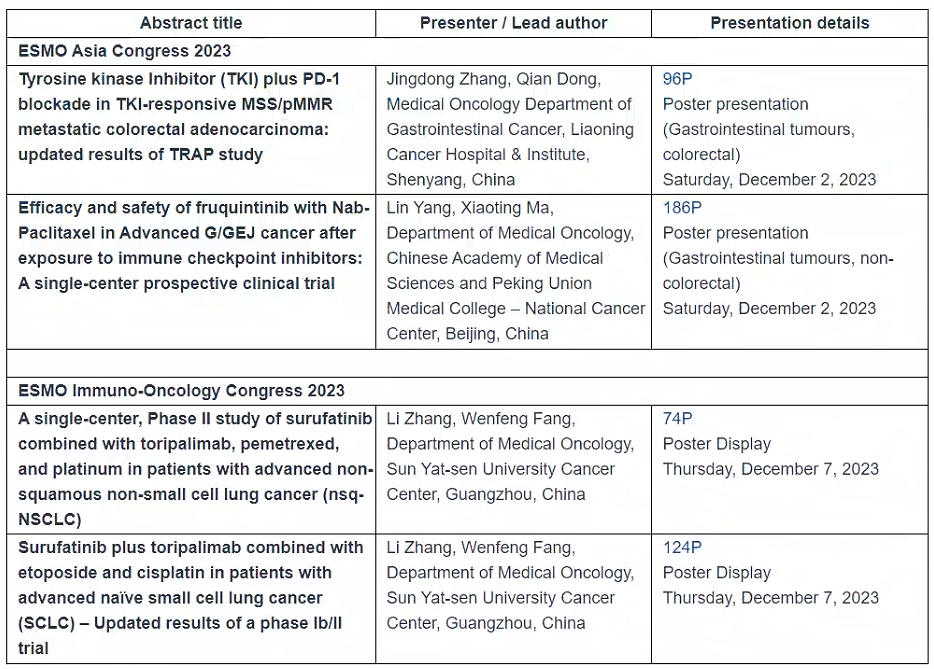
Preview
Source: GlobeNewswire-Health Care
About HUTCHMED
HUTCHMED (Nasdaq/AIM:HCM; HKEX:13) is an innovative, commercial-stage, biopharmaceutical company. It is committed to the discovery and global development and commercialization of targeted therapies and immunotherapies for the treatment of cancer and immunological diseases. It has approximately 5,000 personnel across all its companies, at the center of which is a team of about 1,800 in oncology/immunology. Since inception it has focused on bringing cancer drug candidates from in-house discovery to patients around the world, with its first three medicines marketed in China, the first of which is also marketed in the U.S. For more information, please visit: www.hutch-med.com or follow us on LinkedIn.
The content above comes from the network. if any infringement, please contact us to modify.
Organizations
Drugs
Hot reports
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Leverages most recent intelligence information, enabling fullest potential.



