A rare piece of good news, the world's only drug in development to activate Activated PI3K-delta Syndrome has been approved by the FDA for marketing
31 Mar 2023
Drug ApprovalClinical Study

Preview
Source: SYNAPSE
Recently, Pharming Group N.V. announces that the FDA has approved Joenja® (leniolisib) for the treatment of activated phosphoinositide 3-kinase delta (PI3Kδ) syndrome (APDS) in adult and pediatric patients 12 years of age and older. It becomes the first disease-modifying treatment for the condition, which was discovered just 10 years ago. Patients with APDS have historically been given antibiotics, immunosuppressants and immunoglobulin replacement drugs to deal with the symptoms.
Joenja® is expected to launch in the US in early April and will be available for shipment in mid-April. According to news, Novartis sold rare disease drug leniolisib to Pharming Group N.V for $20 million four years ago.
APDS is a rare primary
immunodeficiency
Activated PI3K-delta Syndrome (APDS) is a rare primary immunodeficiency that was first characterized in 2013 and is
currently estimated to affect 1 to 2 people per million. It is caused by genetic mutations in either the PIK3CD or PIK3R1 genes,
which leads to hyperactivity of the PI3Kδ (phosphoinositide 3-kinase delta) pathway. Balanced signaling in the PI3Kδ pathway is essential for normal
development and function of immune cells in the body. While people with APDS may suffer from a wide variety of symptoms, the most common are frequent and severe infections of the ears, sinuses, and upper and lower respiratory tracts. Infections usually begin in infancy. People with APDS are susceptible to swollen lymph nodes or an enlarged spleen (splenomegaly), as well as autoimmunity and inflammatory symptoms. People with APDS may also be at higher risk for cancers like lymphoma.
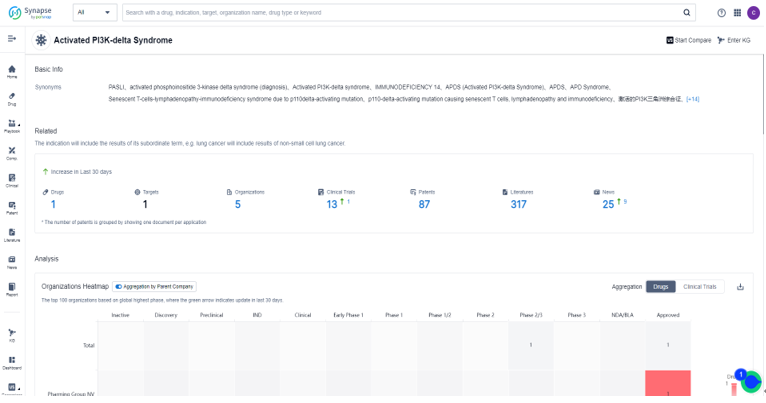
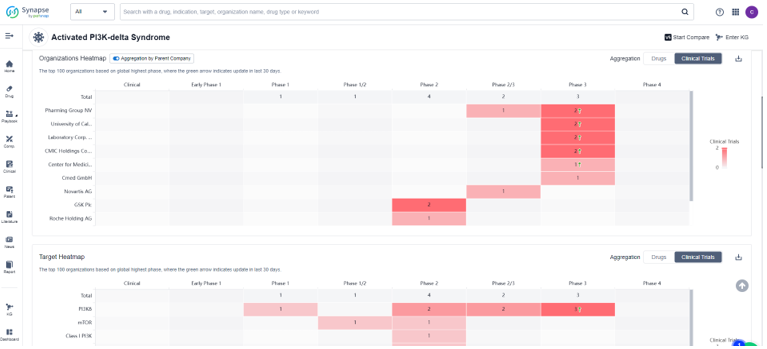
Search the drug intelligence database: Synapse, only 1 drug and 13 clinical trials were found to treat APDS. There are 87 patents、317 literature and 25 news about the indication in the synapse. For this indication clinical trials, the top three R&D organizations are Pharming Group NV, University of California and Laboratory Corp. of America Holdings, the major R&D targets are focused on PI3Kδ、mTOR and Class I PI3K.
Preview
Source: SYNAPSE
Preview
Source: SYNAPSE
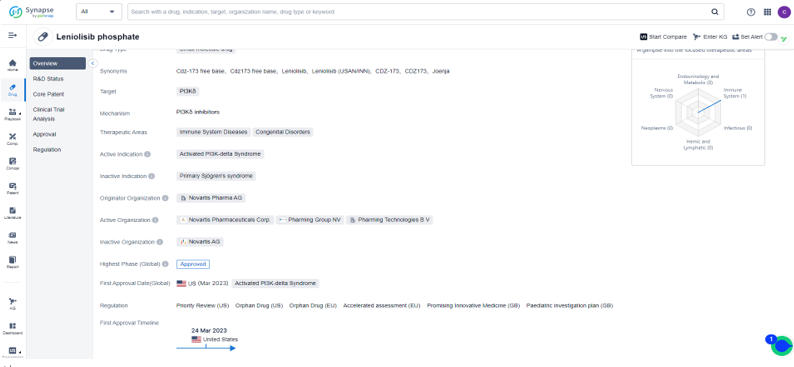
About Joenja®
(Leniolisib)
Joenja® (Leniolisib) is an oral andselective PI3Kδ inhibitor that works to treat APDS by inhibiting the productionof phosphatidylinositol-3-4-5-trisphosphate, which is a cellular messenger that regulates a multitude of cell functions. It is a small molecule drug developed by Novartis Pharma AG, which has recently received its first approval on March 24th, 2023. Leniolisib is a PI3Kδ inhibitor, targeting the delta isoform of the PI3K enzyme, which is involved in various signaling pathways related to cell growth, survival, and inflammation. This drug has been specifically designed for the treatment of Activated PI3K-delta Syndrome, a rare genetic disorder that leads to abnormal immune system function and recurrent infections. By
inhibiting the activity of PI3Kδ, Leniolisib can reduce the activation of immune cells and suppress the inflammatory response in patients with this
syndrome. Overall, Leniolisib represents a promising new treatment option for a rare disease with significant unmet medical needs.
Preview
Source: SYNAPSE
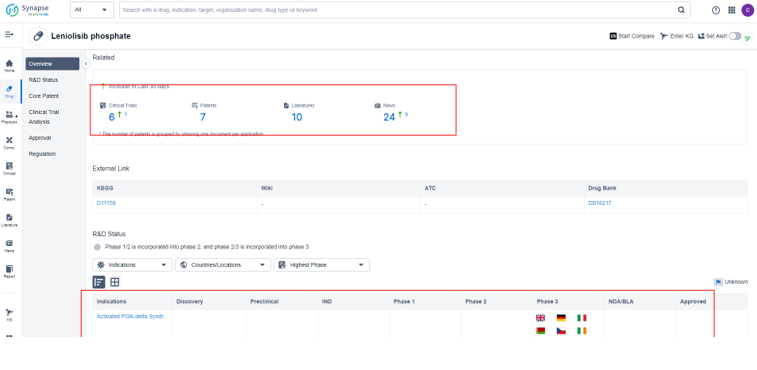
Search the "PatSnap Synapse", the drug is currently laid out in the only one indication "Activated PI3K-delta Syndrome". The global highest R&D Phase was approved in USA recently. In the European Union, the indication is in the NDA/BLA development stage, while in 8 other countries, it
is in Phase 3 clinical trials.
Preview
Source: SYNAPSE
If you’re interested in learning more about this space or keeping track of drug development and clinical trials, sign up for Synapse ( synapse.patsnap.com ) , our freemium product offering.
Organizations
Indications
Targets
Drugs
Hot reports
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Leverages most recent intelligence information, enabling fullest potential.




