AstraZeneca's rare disease add-on therapy Voydeya scores FDA nod
01 Apr 2024
Clinical ResultDrug ApprovalPhase 3Acquisition
Preview
Source: FiercePharma
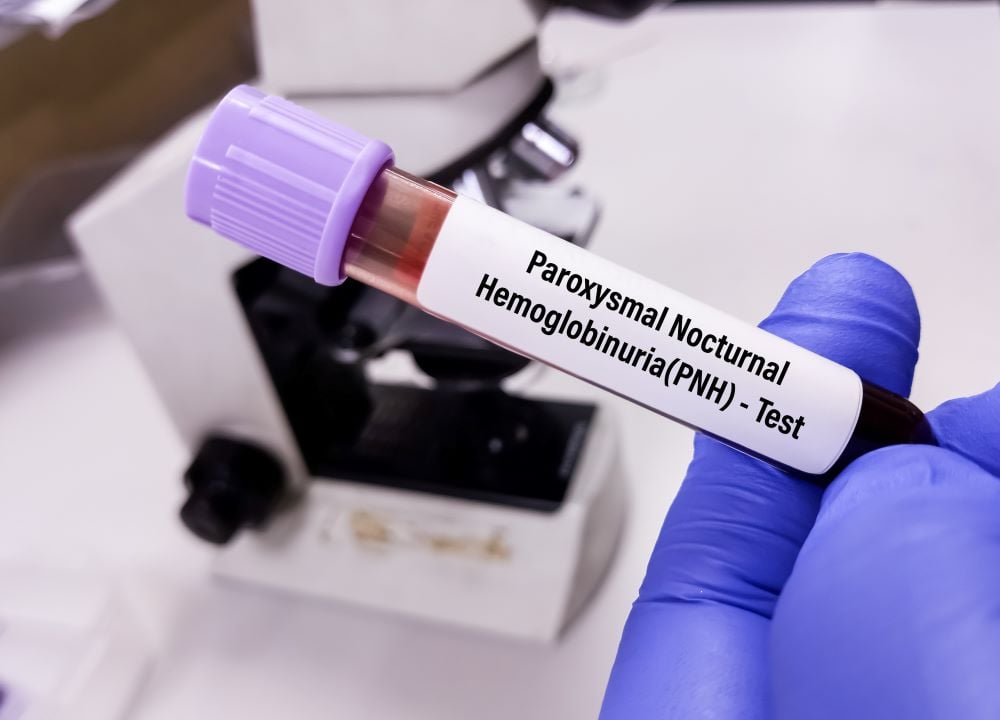
AstraZeneca has strengthened its presence in treating paroxysmal nocturnal hemoglobinuria (PNH) with an FDA approval for Voydeya, an add-on treatment for its PNH standard-of-care drugs Soliris and Ultomiris.
With AstraZeneca’s dominance in the rare blood disease paroxysmal nocturnal hemoglobinuria (PNH) threatened by the recent approval of the first pill on the market for the condition, Novartis’ Fabhalta, AstraZeneca has answered in the United States for its add-on oral treatment in the indication.
The FDA has signed off on Voydeya (danicopan), a factor D inhibitor, to treat PNH patients with extravascular hemolysis (EVH), a condition that causes red blood cell destruction outside of the blood vessels. The nod comes three months after the Japanese Ministry of Health, Labour and Welfare (JHLW) became the first regulator worldwide to endorse the therapy.
AZ said that 10% to 20% of those who use C5 inhibitorsC5 inhibitors such as Soliris and Ultomiris develop EVH, which results in a continuance of anemia symptoms and can require transfusions.
“The approval of Voydeya offers this small subset of PNH patients an add-on therapy designed to address EVH, while maintaining disease control with Ultomiris or Soliris,” Bart Scott, M.D., a professor at the Fred Hutchinson Cancer CenterCancer Center, said in a release.
In the ALPHA phase 3 trial, Voydeya improved hemoglobin levels from baseline to week 12 and met secondary endpoints, including transfusion avoidance.
Long-term follow-up data from the study, which the company presented four months ago, showed that the improvements in mean hemoglobin levels and absolute reticulocyte count levels held up through 48 weeks. Additionally, after 12 weeks, 83% of patients didn’t need a blood transfusion. Through 24 weeks, 78% of patients could say the same.
In February, Europe's regulator recommended Voydeya for approval. AZ also is investigating danicopan as a treatment for the macular degeneration condition geographic atrophy.
In addition to having a convenience edge as an oral treatment over the infused C5 inhibitorsC5 inhibitors, Novartis’ factor B inhibitor Fabhalta may have an advantage in preventing red blood cell destruction as it works upstream of the C5 terminal pathway.
Another new competitor in PNH is Appelis’ C3 inhibitor Empaveli which targets a different enzyme than AZ’s treatments. Empavelli generated sales of $91 million in 2023, which was its second full year on the market.
For more details,please visit the original website
The content of the article does not represent any opinions of Synapse and its affiliated companies. If there is any copyright infringement or error, please contact us, and we will deal with it within 24 hours.
Organizations
Indications
Targets
Drugs
Hot reports
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Leverages most recent intelligence information, enabling fullest potential.