New five-year data on disability outcomes and safety of Ofatumumab in patients with relapsing multiple sclerosis presented by novartis
23 Apr 2023
Clinical ResultAntibody
PatSnap Synapse, a novel, AI Powered Drug Competitive Intelligence database. Access to drugs, clinical trials, patents, literature and news in one search.
Novartis AG, a Swiss-based multinational pharmaceutical company, has recently presented new 5-year data on the safety and disability outcomes of its monoclonal antibody drug, Ofatumumab, for people living with relapsing multiple sclerosis (RMS). The drug targets CD20, which is found on the surface of B-lymphocytes, and works by inhibiting CD20, leading to complement-dependent cytotoxicity (CDC) effects and antibody-dependent cellular cytotoxicity (ADCC) against B-cells.
The 5-year data presented by Novartis AG showed that patients treated earlier and continuously with Kesimpta® (ofatumumab) had fewer disability worsening events and low brain volume change versus those who started on teriflunomide and were later switched to Kesimpta. A separate analysis showed that treatment with Kesimpta for up to five years was well-tolerated, with no new or increased safety risks identified.
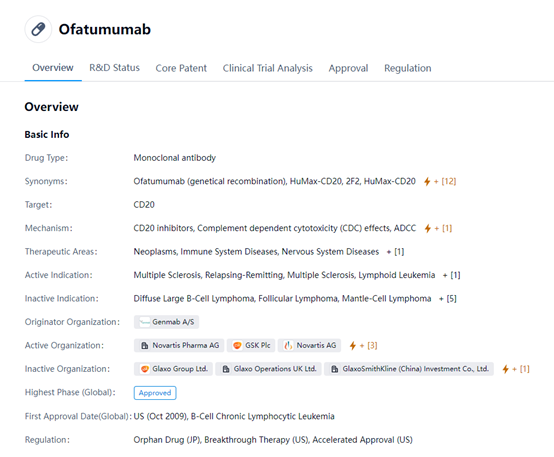
Ofatumumab is a monoclonal antibody drug that targets CD20, a protein found on the surface of B-lymphocytes. The drug works by inhibiting CD20, leading to complement-dependent cytotoxicity (CDC) effects and antibody-dependent cellular cytotoxicity (ADCC) against B-cells.
Ofatumumab is used to treat multiple sclerosis, both relapsing-remitting and lymphoid leukemia. It has been approved for use in the United States since October 2009 for the treatment of B-cell chronic lymphocytic leukemia. Ofatumumab has also been tested for the treatment of diffuse large B-cell lymphoma, follicular lymphoma, and mantle-cell lymphoma, but did not show significant benefits for these indications. The therapeutic areas for Ofatumumab include neoplasms, immune system diseases, and nervous system diseases. The drug was originally developed by Genmab A/S and is now actively developed and marketed by Novartis AG.
Preview
Source: SYNAPSE
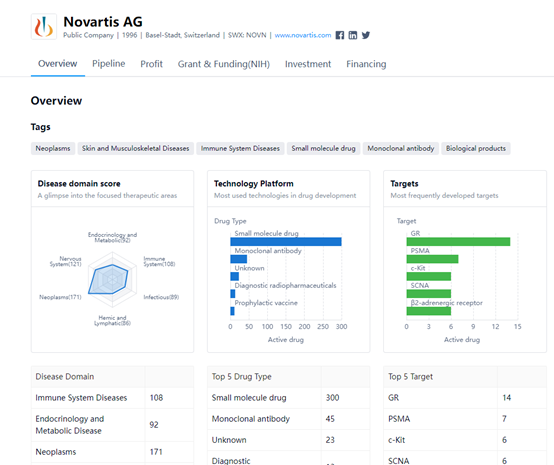
Novartis AG is focused on developing drugs for neoplasms, nervous system diseases, immune system diseases, endocrinology and metabolic disease, infectious diseases, and hemic and lymphatic diseases. The company's drug development technologies include small molecule drugs,
monoclonal antibodies, diagnostic radiopharmaceuticals, and prophylactic vaccines. Some of the most frequently developed targets by Novartis AG include GR, PSMA, c-Kit, SCNA, and the β2-adrenergic receptor.
Preview
Source: SYNAPSE
In conclusion, the new 5-year data presented by Novartis AG on the safety and disability outcomes of Ofatumumab for RMS patients is promising. As Novartis AG continues to focus on developing innovative drugs for a variety of therapeutic areas, Ofatumumab remains a valuable treatment option for patients living with RMS.
Organizations
Indications
Hot reports
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Leverages most recent intelligence information, enabling fullest potential.