Septerna raises $150m in Series B funding round
12 Jul 2023
PROTACs
Preview
Source: Pharmaceutical Technology
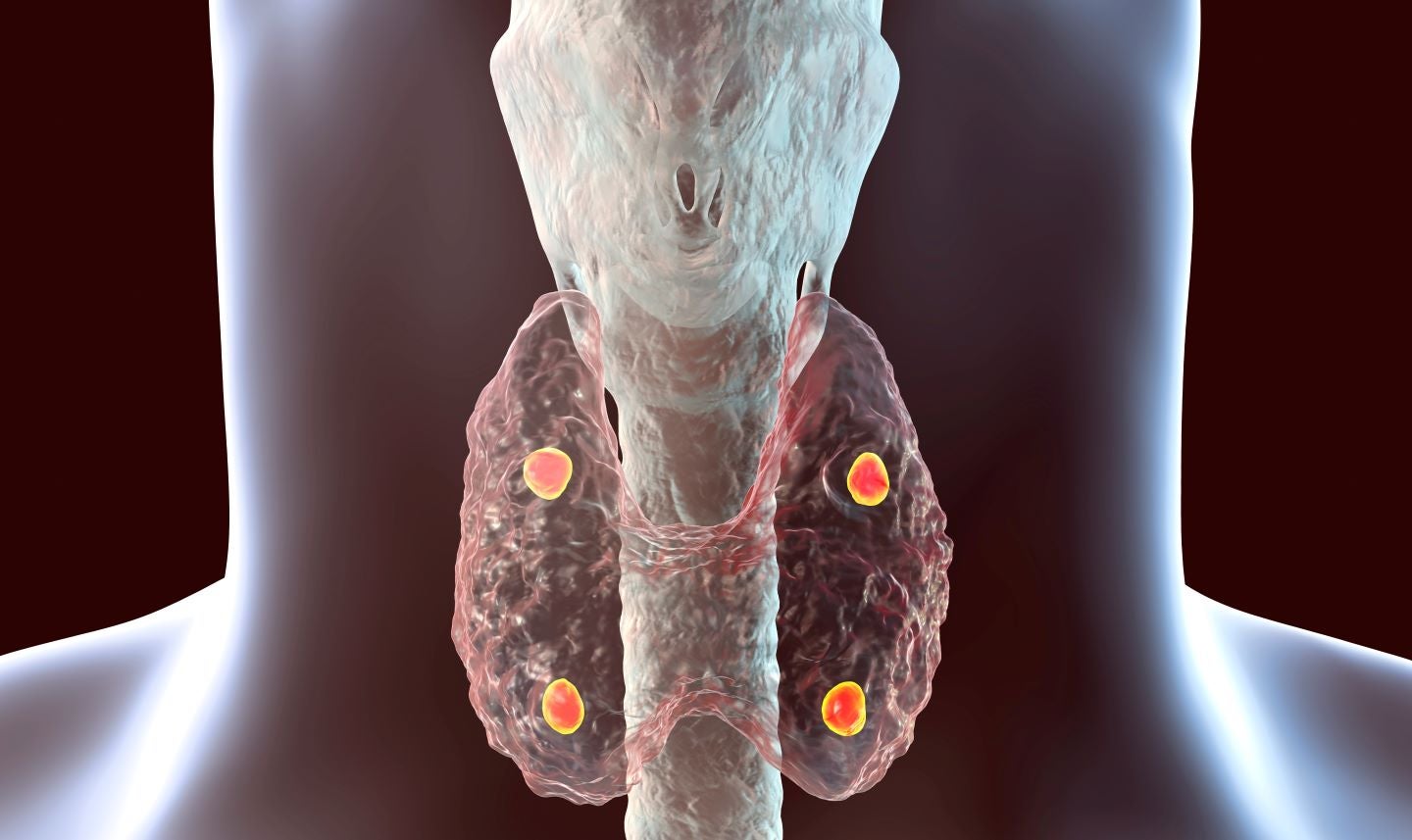
A PTH1R agonistPTH1R agonist programme of Septerna is being developed to treat hypoparathyroidism. Credit: Kateryna Kon via Shutterstock.com.
Biotechnology firm Septerna has raised $150m in a Series B funding round to develop its pipeline of new oral small molecule therapies that act on G protein-coupled receptors (GPCRs).
New investor RA Capital Management led the round, with current investors Third Rock Ventures, Invus, Catalio Capital Management, Samsara BioCapital and BVF Partners among others taking part.
Recommended Reports

Preview
Source: Pharmaceutical Technology
ReportsLOA and PTSR Model - Triplex in Myelofibrosis GlobalData

Preview
Source: Pharmaceutical Technology
ReportsLOA and PTSR Model - AVID-200 in Myelofibrosis GlobalData
View allCompanies IntelligenceSamsara BioCapital LLCThird Rock Ventures LLCRA Capital Management LPBVF Partners LPCatalio Capital Management LPView all
RA Capital Management partner Jake Simson will join the board of directors of Septerna.
The proceeds will be utilised for portfolio development and to progress its lead parathyroid hormone 1 receptor (PTH1R) programme to clinical proof-of-mechanism.
This PTH1R agonistPTH1R agonist programme is being developed to treat hypoparathyroidism, a condition marked by reduced levels of PTH.
The company will also use the funds to advance the preclinical development of a second thyroid-stimulating hormone receptor-targeting programme and other initial-stage assets.
“This is an exciting time for GPCR drug development, and we are eager to move our novel products toward clinical development.”
For more details,please visit the original website
The content of the article does not represent any opinions of Synapse and its affiliated companies. If there is any copyright infringement or error, please contact us, and we will deal with it within 24 hours.
Organizations
Indications
Chat with Hiro
Hot reports
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.





